0549
Investigating the contribution of hyaluronan (HA) to the breast tumour microenvironment using multiparametric MRI and histology1Radiotherapy & Imaging, Institute of Cancer Research, London, United Kingdom, 2Royal Marsden NHS Foundation Trust, London, United Kingdom, 3Halozyme Therapeutics, San Diego, CA, United States, 4Life Sciences and Medicine, King's College London, London, United Kingdom
Synopsis
The impact of HA on tumour characteristics measured by MRI/E is not fully understood. We evaluated relationships between MRI biomarkers and histology in saline (HA present) and PEGPH20 treated (HA absent) breast tumours. ADC had the strongest association with HA, reaffirming that ADC is the most sensitive biomarker of tumour response to PEGPH20. MTR negatively correlated with HA, likely due to a HA-mediated dilution of MT-inducing components including collagen. MRE-derived elasticity (Gd) did not directly correlate with HA, however, HA degradation reduced the contribution of collagen to tumour viscoelasticity and may shift the sensitivity of Gd to the cellular compartment.
Introduction
Hyaluronan (HA) and collagen contribute to the dense extracellular matrix (ECM) associated with breast cancer[1]. Stromal-modulating therapies are being developed that target the tumour ECM for therapeutic gain. PEGPH20, a recombinant, PEGylated, human hyaluronidase, can degrade HA and improve pre-clinical tumour response to therapy[2-5]. We previously showed that the apparent diffusion coefficient (ADC), estimated from diffusion weighted imaging (DWI), is a sensitive biomarker of pre-clinical breast tumour response to PEGPH20, likely due to loss of water from the tumour mass and collapse of the extracellular space following HA degradation[6]. Additional MRI biomarkers, including magnetisation transfer ratio (MTR), elasticity (Gd), and viscosity (Gl), were evaluated but failed to provide robust biomarkers of tumour response[6-8].To further interrogate the impact of HA on MRI biomarkers and the underlying tumour microenvironment, we examined the relationships between MRI biomarkers (MTR, ADC, Gd, and Gl) and histology in three pre-clinical breast tumour models, and investigated how these associations differ in the presence and absence of HA.
Methods
Experiments were performed in accordance with the UK Animals (Scientific Procedures) Act 1996. Murine 4T1 (n = 12) and HA synthase 3 overexpressing 4T1 (4T1/HAS3; n = 24) breast tumours were propagated orthotopically in female BALB/c mice. Human luc-MDA-MB-231 LM2-4 breast tumours (n = 24) were propagated orthotopically in female athymic NCr-Foxn1nu mice.Multiparametric MRI was performed 24 hours after treatment with either saline or PEGPH20 (1 mg/kg) on a Bruker 7T horizontal MRI system as previously described[6, 8, 9]. MRI included anatomical T2-weighted imaging, MT-RARE to estimate MTR, DWI for the ADC, and MR elastography (MRE) to estimate Gd and Gl. Parametric maps of MTR and ADC were reconstructed from a 1 mm thick axial slice over a 30 x 30 mm FOV, whilst Gd and Gl were reconstructed isotropically from three 0.3 mm thick axial slices over a 19.2 x 19.2 mm FOV. Median values for each tumour were calculated from a region of interest which covered viable tumour tissue and excluded necrosis.
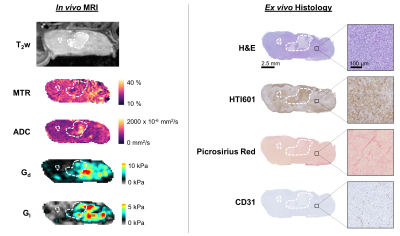
MRI-aligned FFPE tissue sections (5 μm) were stained with H&E, HTI601[10] (HA), picrosirius red (collagen I & III), or CD31 (blood vessels). Nuclear density, percent HA, and blood vessel density were quantified from H&E, HTI601 and CD31 staining respectively using Definiens Tissue Studio®. Percent collagen was quantified as previously described[11].
Statistical analyses were performed using Prism 9 (GraphPad) with a significance level of 5%.
Results & Discussion
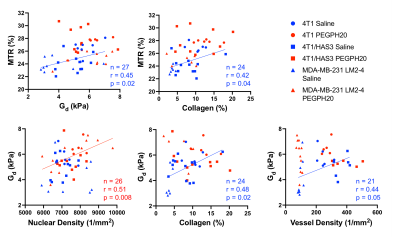
Multiparametric MRI and histology images for one representative saline control MDA-MB-231 LM2-4 breast tumour are shown in Figure 1. Clear spatial correspondence between the MRI and histology is apparent, enabling assessment of the pathological determinant(s) of the MRI biomarkers.The correlation matrix for all the post-treatment (saline & PEGPH20) measurements is shown in Figure 2a. ADC exhibited the strongest correlation with percent HA (n = 56, r = 0.53, p = 0.00003), reaffirming that ADC is the most sensitive biomarker of tumour response to PEGPH20[6, 12]. MTR negatively correlated with HA (n = 57, r = -0.46, p = 0.0003) likely because HA-mediated fluid retention and extracellular expansion will regulate the amount of free water and dilute the effect of MT-inducing components such as collagen.
Surprisingly, collagen did not appear to be a major determinant of breast tumour viscoelasticity as we previously demonstrated[11]. This prompted us to further investigate the contribution of HA to Gd and Gl by separating the data into saline control (HA present; Figure 2b) and PEGPH20 treated tumours (HA absent; Figure 2c).
When HA was present, Gd positively correlated with MTR (Figures 2b & 3; n = 27, r = 0.45, p = 0.02), Gd and MTR positively associated with collagen content (Gd: n= 24, r = 0.48, p = 0.02; MTR: n = 24, r = 0.42, p = 0.04), and Gd positively correlated with vessel density (n = 21, r = 0.44, p = 0.05). The positive correlation between Gd and collagen corroborated our previous study showing the important contribution of collagen to tumour viscoelasticity[11]. However, in tumours where HA was degraded by PEGPH20, the associations between Gd, MTR, collagen, and vessel density disappeared. In the absence of HA, Gd positively correlated with nuclear density (Figures 2c & 3; n = 26, r = 0.51, p = 0.008). The lack of correlation between Gd and collagen in PEGPH20 treated tumours suggests that HA degradation directly affects the structure-function of collagen or more generally the mechanical integrity of the ECM, shifting the sensitivity of Gd towards the remaining intact cellular network.
Conclusion
In conclusion, our data support ADC as the most sensitive biomarker of HA degradation due to its sensitivity to change in size of the extracellular compartment. MTR is a sensitive yet less specific biomarker of HA accumulation and its therapeutic modulation, as HA is associated with a larger extracellular space and increased free water content, thereby reducing the proportion of MT-inducing collagen-bound water in the tumour. While HA does not directly affect Gd or Gl, HA degradation can reduce the contribution of collagen to tumour viscoelastic properties, likely due to a loss of connection between HA and other ECM components, resulting in a loosening of the fibril network.Acknowledgements
We acknowledge support from the Cancer Research UK Centre at the ICR, European Union Horizon 2020 Research and Innovation Programme (Grant #668039), the Rosetrees Trust (M593), Cancer Research UK support to the Cancer Imaging Centre at the ICR, in association with the MRC and Department of Health (England) (C1060/A16464), Cancer Research UK grant C16412/A27725 and a Children with Cancer UK Research Fellowship.References
1. Insua-Rodríguez, J. and T. Oskarsson, The extracellular matrix in breast cancer. Advanced Drug Delivery Reviews, 2016. 97(Supplement C): p. 41-55.
2. Provenzano, P.P., et al., Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 2012. 21(3): p. 418-29.
3. Thompson, C.B., et al., Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther, 2010. 9(11): p. 3052-64.
4. Clift, R., et al., Remodeling the Tumor Microenvironment Sensitizes Breast Tumors to Anti-Programmed Death-Ligand 1 Immunotherapy. Cancer Research, 2019. 79(16): p. 4149.
5. Bahn, J.D., et al., Abstract 2501: PEGylated human recombinant hyaluronidase (PEGPH20) enhances radiotherapy in hyaluronan-rich solid tumors. Cancer Research, 2011. 71(8 Supplement): p. 2501.
6. Reeves, E.L., et al. 0143 Breast tumour response to PEGPH20-induced stromal modulation assessed by multiparametric MRI. in Proc. Intl. Soc. Mag. Reson. Med. 28. 2020.
7. Reeves, E.L., et al. 4850 Intrinsic susceptibility MRI can inform on tumour vascular decompression in response to the stromal targeted therapy PEGPH20. in Proc. Intl. Soc. Mag. Reson. Med. 28. 2020.
8. Reeves, E.L., et al., 0930: MR elastography reveals a marked increase in breast cancer viscoelasticity in vivo following hyaluronan degrdation by PEGPH20. Proc. Intl. Soc. Mag. Reson. Med. 29., 2021.
9. Li, J., et al., Tumour biomechanical response to the vascular disrupting agent ZD6126 in vivo assessed by magnetic resonance elastography. British Journal of Cancer, 2014. 110(7): p. 1727-1732.
10. Jadin, L., et al., Characterization of a Novel Recombinant Hyaluronan Binding Protein for Tissue Hyaluronan Detection. Journal of Histochemistry & Cytochemistry, 2014. 62(9): p. 672-683.
11. Li, J., et al., Investigating the Contribution of Collagen to the Tumor Biomechanical Phenotype with Noninvasive Magnetic Resonance Elastography. Cancer Research, 2019.
12. Arias-Lorza, A.M. and N. Raghunand, 0949: ADC Decreases in Solid Tumors Following Monotherapy With PEGylated Recombinant Human Hyaluronidase: Results From Early-Phase Clincal Trials. Proc. Intl. Soc. Mag. Reson. Med. 29., 2021.
Figures