0536
On the robustness of single UTE-Dixon for simultaneous short T2*, water and fat imaging across skeletal anatomies
Sophia Kronthaler1, Georg C. Feuerriegel1, Christof Boehm1, Alexandra S. Gersing1, Benedikt J. Schwaiger2, Marcus R. Makowski1, Kilian Weiss3, and Dimitrios C. Karampinos1
1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Department of Neuroradiology, Technical University of Munich, Munich, Germany, 3Philips GmbH Market DACH, Hamburg, Germany
1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Department of Neuroradiology, Technical University of Munich, Munich, Germany, 3Philips GmbH Market DACH, Hamburg, Germany
Synopsis
In patients with vertebral fractures or degenerative changes often CT and MR imaging are performed, with CT aiming at the characterization of osseous changes and the MR focusing on soft tissue components. Previous work presented a single UTE-Dixon (sUTE-Dixon) approach which enabled the simultaneous assessment of vertebral fractures and edema of the thoracolumbar spine from a single ultrashort-echo time (UTE) image. This work investigates the robustness of the sUTE-Dixon methodology in various skeletal anatomies.
Introduction & Purpose
CT and MR imaging are often performed in patients with bone fractures or degenerative changes to assess the osseous and soft tissue components (1-3). In the case of an acute fracture, the CT aims to identify the fracture lines for potential surgery planning and MR imaging may be additionally performed to identify edema (2). However, CT examinations, across anatomical locations, are associated with radiation exposure, additional examination time, and costs. Ultrashort-echo time (UTE) imaging enables signal detection from tissues with short T2* components, such as cortical bone. It was shown that UTE imaging enables the morphological assessment of fractures and degenerative bone changes in the spine (4,5) and joints (5,6). Previous work presented a single UTE-Dixon approach which enabled the simultaneous assessment of vertebral fractures and edema of the thoracolumbar spine from a single ultrashort-echo time image (7,8). The proposed sUTE-Dixon methodology removed unwanted low-frequency background phases to allow water-fat separation and SWI processing from a complex sUTE image (8) in the thoracolumbar spine. By applying the sUTE-Dixon method in different skeletal anatomies, the present work shows that the method can be applied in regions with strong B0 inhomogeneities and reliably generate short T2*, water and fat images for different anatomies such as the knee, the mandible, and the whole spine.Methods
In vivo 3D-UTE measurements were performed with a stack-of-stars center-out radial acquisition and phase-encoding in the third cartesian dimension on a 3T system (Figure 1). All scans were SENSE accelerated in the cartesian dimension. The contrast of the UTE magnitude images was inverted such that bone appears bright (4,9).The UTE phase images contain unwanted phase components ϕ(t) which arise due to B0 inhomogeneities, eddy currents, signal delays in the receiver chains, and the B1 transmit/receive phase. Water and fat maps were obtained by solving the following smoothness-constrained non-linear inverse water-fat problem for the unwanted phase term ϕ (8):
$$ \phi=argmin_{\phi}\;\psi(\phi)=argmin_{\phi}\frac{1}{2}\left\|(W+Fe^{i\theta(TE)})e^{i\phi}-S(TE))\right\|^2_2+\lambda\left\|M\nabla\phi\right\|^2_2 $$
Results
In the inverted magnitude images of a high-resolution UTE scan of the knee, small cortical bone structures were well depicted (Figure 2). The ϕ images showed a larger contribution from B0 phase terms compared to B1 transmit/receive phase. With a regularization parameter of λ=5, high-quality water and fat separated images were obtained. A scan of a volunteer’s mandible depicted a high B1 transmit-receive phase contribution (Figure 3) which was demodulated. The noise level of the Water/Fat-images was higher compared to other anatomies, however, small fatty areas were well depicted. Within the spine, SNR was too low to achieve a good separation within the vertebras. Using a larger TE (0.4 ms) and Head Coil with an additional Neck Coil resulted in higher SNR in the cervical spine (Figure 4) and resulted also in better water and fat images. The cervical, compared to the thoracic spine, was strongly affected by B0 inhomogeneities. In the UTE spDixon water images, there was signal within the anterior subcutaneous fat region, which is prone to artifacts due to abdominal breathing.Discussion & Conclusion
In our study of different MSK anatomies, we showed that the sUTE-Dixon processing allowed the removal of low-frequency background phases, enabling reliable water-fat separation from a complex sUTE image. The study was performed in five anatomical locations, on two different scanner systems, with various coils and varying echo times. In all cases, a good depiction of short T2* components and good water and fat separation was achieved. Especially the cervical spine yields higher changes in B0 inhomogeneities that were still successfully demodulated. For the knee scans, a transmit-receive coil was used which resulted in a different B1 transmit-receive phase when compared to receive-only coils. Despite this additional challenge, the presented methodology was able to provide high-quality water and fat images.The limitations of this study were, firstly that the regularization parameter was not the same across all anatomies and was manually adjusted by visual inspection of the water-fat images. However, the regularization parameter had to be tuned only once for each anatomy. Secondly, the water-fat separation can be challenging in areas affected by motion such as abdominal breathing.
Acknowledgements
The present work was supported by the European Research Council (grant agreement No 677661) and Philips Healthcare.References
- Shanechi AM, Kiczek M, Khan M, Jindal G. Spine Anatomy Imaging: An Update. Neuroimaging Clin N Am 2019;29(4):461-480.
- Piazzolla A, Solarino G, Lamartina C, De Giorgi S, Bizzoca D, Berjano P, Garofalo N, Setti S, Dicuonzo F, Moretti B. Vertebral Bone Marrow Edema (VBME) in Conservatively Treated Acute Vertebral Compression Fractures (VCFs): Evolution and Clinical Correlations. Spine (Phila Pa 1976) 2015;40(14):E842-848.
- Mandalia V, Henson JH. Traumatic bone bruising--a review article. Eur J Radiol 2008;67(1):54-61.
- Schwaiger BJ, Schneider C, Kronthaler S, Gassert FT, Bohm C, Pfeiffer D, Baum T, Kirschke JS, Karampinos DC, Makowski MR, Woertler K, Wurm M, Gersing AS. CT-like images based on T1 spoiled gradient-echo and ultra-short echo time MRI sequences for the assessment of vertebral fractures and degenerative bone changes of the spine. Eur Radiol 2021;31(7):4680-4689.
- Argentieri EC, Koff MF, Breighner RE, Endo Y, Shah PH, Sneag DB. Diagnostic Accuracy of Zero-Echo Time MRI for the Evaluation of Cervical Neural Foraminal Stenosis. Spine (Phila Pa 1976) 2018;43(13):928-933.
- Breighner RE, Bogner EA, Lee SC, Koff MF, Potter HG. Evaluation of Osseous Morphology of the Hip Using Zero Echo Time Magnetic Resonance Imaging. Am J Sports Med 2019;47(14):3460-3468.
- Gersing AS, Bodden J, Neumann J, Diefenbach MN, Kronthaler S, Pfeiffer D, Knebel C, Baum T, Schwaiger BJ, Hock A, Rummeny EJ, Woertler K, Karampinos DC. Accelerating anatomical 2D turbo spin echo imaging of the ankle using compressed sensing. Eur J Radiol 2019;118:277-284.
- Kronthaler S, Boehm C, Feuerriegel G, Bornert P, Katscher U, Weiss K, Makowski MR, Schwaiger BJ, Gersing AS, Karampinos DC. Assessment of vertebral fractures and edema of the thoracolumbar spine based on water-fat and susceptibility-weighted images derived from a single ultra-short echo time scan. Magn Reson Med 2021.
- Breighner RE, Endo Y, Konin GP, Gulotta LV, Koff MF, Potter HG. Technical Developments: Zero Echo Time Imaging of the Shoulder: Enhanced Osseous Detail by Using MR Imaging. Radiology 2018;286(3):960-966.
Figures
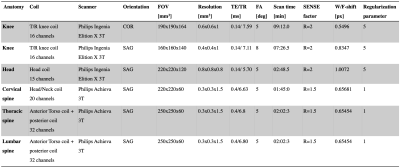
Figure 1. Table of the used scan parameters including the different regularization parameters.
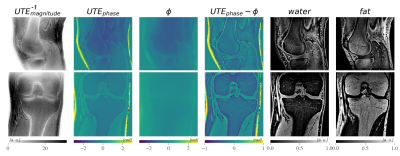
Figure 2. Coronal and sagittal high-resolution scans of a healthy volunteer’s knee. The inverted UTE magnitude images visualized thin cortical bone structures. In the ϕ images phase modulations due to B0 inhomogeneities were visible especially in the sagittal view.
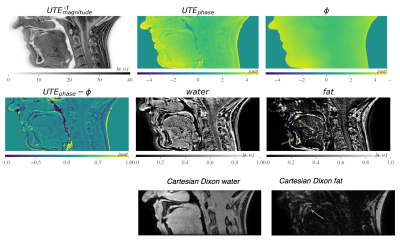
Figure 3. 3D isotropic UTE scan of a healthy volunteer’s mandible. Within the mandible fat was well depicted when compared to a Cartesian multi-echo Dixon fat map (green arrows).
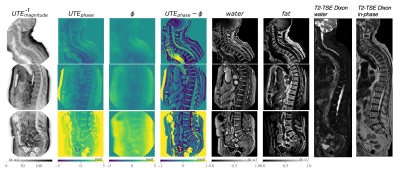
Figure 4. High-resolution full spine scan of a patient. The cervical, thoracic and lumbar spine were acquired in three separate scans. A T2-TSE Dixon water and in-phase image were acquired as a reference. The ϕ image of the cervical spine shows strong phase contributions due to B0 field inhomogeneities that were caused by the bent anatomy. The anterior subcutaneous fat region was prone to errors, which were affected by abdominal breathing.
DOI: https://doi.org/10.58530/2022/0536