0534
Using machine learning to build a population model of tongue muscle architecture based on mDIXON and diffusion tensor imaging
Robert Lloyd1,2, Iain Ball3, and Lynne Bilston1,2
1Neuroscience Research Australia, Sydney, Australia, 2University of New South Wales, Sydney, Australia, 3Philips Australia & New Zealand, Sydney, Australia
1Neuroscience Research Australia, Sydney, Australia, 2University of New South Wales, Sydney, Australia, 3Philips Australia & New Zealand, Sydney, Australia
Synopsis
Simulations of obstructive sleep apnoea (OSA) require detailed models of the muscle fibres within the tongue, to correctly capture the motion of the tongue. Diffusion weighted images (DWI) of the oral cavity were collected for 5 healthy controls. Fibre-orientation distributions (FOD) estimated from subject DWI, can resolve intersecting muscles in the tongue. The group averaged FOD reduced the influence of spurious peaks, which gave clearer boundaries between the intrinsic muscles of the tongue. These results may help fully automate the segmentation of the muscles within the tongue.
Introduction
Obstructive sleep apnoea (OSA) is a respiratory disorder characterised by the repetitive partial, or complete closure of the upper airway, reducing air flow during sleep. Coordinated anterior motion of the tongue is needed to maintain airway patency during inspiration1, and the magnitude of the displacement required depends on individual anatomy1, and the position of the head2. Subject-specific models can help improve the current understanding of sleep apnoea, as they can isolate the effect of different factors within the population such as variation in anatomy or neural activation. To develop these models, the fibre structure of the muscles in the tongue are required to delineate intersecting muscles and simulate the correct motion. In this work, we aim to produce template model of the muscle architecture, to automate the segmentation of the intrinsic and extrinsic tongue muscles, for subject-specific computational models.Methods
The oral cavity of five healthy control participants (3 females, and 2 males, aged 28-33 years old) were scanned in a 3T Philips Ingenia CX (Philips Healthcare, Best, The Netherlands), using the 16-channel neurovascular coil. Participants lay on the scanning bed supine, with the Frankfort plane vertical, and asked to refrain from swallowing during scans. Anatomical images were acquired with a two-point mDIXON fast field echo (FFE) scan. 170 sagittal slices were collected, coving the head with the tongue centred in the foot-head direction. Imaging parameters include: TR/TE1/TE2 = 4.15/1.19/2.37 ms, FOV = 240×240 mm, slice thickness = 1 mm, in-plane resolution =0.938 mm. Diffusion images were acquired with a single shot EPI sequence with 49 directions (1 B0 volume, 48 b-value = 700 s.mm-2 volume) sampled evenly over a full-sphere, and optimised for gradient load. The field of view was centred over the tongue and a ~40 mm thick rest-slab supressed the posterior of the head and neck. Imaging parameters include: TR/TE = 4671/54 ms, FOV = 156×192×81mm, resolution 3 mm isotropic, with an optimized SPAIR pulse and slice-selection gradient reversal (SSGR) for fat suppression. Eddy-current correction was performed on-line during the image reconstruction. A previously trained U-Net3 model used all contrast modes of mDIXON scans to automatically segment the oral cavity. These labels were used to mask the tongue throughout analysis. In MRtrix3 all images were resampled with an isotropic resolution of 1.5 mm. For all subjects the constrained spherical deconvolution (CSD) response function of a single fibre was estimated4, and used to calculate the fibre-orientation distribution (FOD) for the whole tongue5. Diffeomorphic registration6 with multiple contrast images (in-phase/out-phase mDIXON, and FOD map) was used to align the participant’s oral cavities, and find the group average FOD. Each subjects inverse non-linear warp was then used to fit the group average FOD to their anatomy. Probabilistic fibre tracking was performed on each subjects FOD7, and fitted average FOD to visualise the muscle fibre directions, and reducing the influence of spurious FOD peaks. Spherical-deconvolution informed filtering was then used to improve the fit of the generated streamlines8.Results
Figure 1 shows the filtered muscle fibre tracts overlaid on the in-phase mDIXON image of a representative subject. Figure 2 shows the tracts estimated from the average model fitted to the same subject. In the average model there are clearer boundaries between muscles of the tongue, particularly between the genioglossus/combined longitudinal and between the transverse/vertical muscles. However, the thickness of the transverse and mylohyoid muscles where over and underestimated by the fitted model, respectively.Discussion
This study provides a proof of concept that a semi-automated imaging and analysis pipeline can reconstruct the complex structure of the muscles in the tongue. The averaged FOD reduced the influence of spurious peaks during fibre tracking, which resulted in clearer boundaries between the intrinsic muscles of the tongue, than with the FOD of a single subject. The FOD peaks provide structural information that allow a trained U-Net model to fully automate the segmentation of the muscles of the tongue. A larger subject cohort would enable the model to be generalised to the broader population. Diffusion images with multiple b-values have also been acquired, to assess whether spurious peaks are reduced during FOD estimation, to improve the fibre model.Acknowledgements
L.E.B. is supported by an Australian National Health and Medical Research Council (NHMRC) investigator grant (APP1172988). This work and R.A.L are funded by an Australian Research Council (ARC) grant (DP200100211). I.K.B. is affiliated with Philips Australia & New Zealand.References
1. Cheng, S.; Brown, E. C.; Hatt, A.; Butler, J. E.; Gandevia, S. C.; Bilston, L. E., Healthy Humans with a Narrow Upper Airway Maintain Patency During Quiet Breathing by Dilating the Airway During Inspiration. The Journal of physiology 2014, 592, 4763-4774.2. Cai, M.; Brown, E. C.; Hatt, A.; Cheng, S.; Bilston, L. E., Effect of Head and Jaw Position on Respiratory-Related Motion of the Genioglossus. Journal of Applied Physiology 2016, 120, 758-765.
3. Isensee, F.; Petersen, J.; Klein, A.; Zimmerer, D.; Jaeger, P. F.; Kohl, S.; Wasserthal, J.; Koehler, G.; Norajitra, T.; Wirkert, S., Nnu-Net: Self-Adapting Framework for U-Net-Based Medical Image Segmentation. arXiv preprint arXiv:1809.10486 2018.
4. Tournier, J. D.; Calamante, F.; Connelly, A., Determination of the Appropriate B Value and Number of Gradient Directions for High‐Angular‐Resolution Diffusion‐Weighted Imaging. NMR in Biomedicine 2013, 26, 1775-1786.
5. Jeurissen, B.; Tournier, J.-D.; Dhollander, T.; Connelly, A.; Sijbers, J., Multi-Tissue Constrained Spherical Deconvolution for Improved Analysis of Multi-Shell Diffusion Mri Data. NeuroImage 2014, 103, 411-426.
6. Raffelt, D.; Tournier, J.-D.; Fripp, J.; Crozier, S.; Connelly, A.; Salvado, O., Symmetric Diffeomorphic Registration of Fibre Orientation Distributions. NeuroImage 2011, 56, 1171-1180.
7. Tournier, J. D.; Calamante, F.; Connelly, A. In Improved Probabilistic Streamlines Tractography by 2nd Order Integration over Fibre Orientation Distributions, Proceedings of the international society for magnetic resonance in medicine, John Wiley & Sons, Inc. New Jersey, USA: 2010.
8. Smith, R. E.; Tournier, J.-D.; Calamante, F.; Connelly, A., Sift: Spherical-Deconvolution Informed Filtering of Tractograms. NeuroImage 2013, 67, 298-312.
Figures
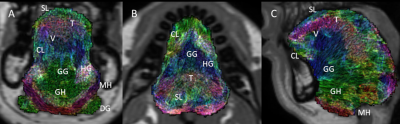
Filtered tongue
muscle fibre tracts of a representative subject, overlaid on the subject’s
anatomical scan. A-C show the coronal, axial, and midsagittal slices of the
tongue. Annotations indicate the separation of individual muscles. GG:
genioglossus, GH: geniohyoid, MH: mylohyoid, HG: hyoglossus, SL: superior
longitudinal, CL: combined longitudinal, V: vertical, T: transverse, DG:
digastric.
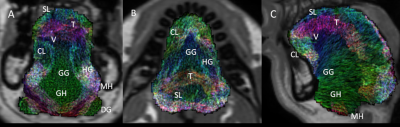
Filtered
tongue muscle fibre tracts of the group average fitted to a representative
subject, overlaid on the subject’s anatomical scan. A-C show the coronal,
axial, and midsagittal slices of the tongue. Annotations indicate the
separation of individual muscles. GG: genioglossus, GH: geniohyoid, MH:
mylohyoid, HG: hyoglossus, SL: superior longitudinal, CL: combined
longitudinal, V: vertical, T: transverse, DG: digastric.
DOI: https://doi.org/10.58530/2022/0534