0530
The relationship between cytochrome oxidase redox state and CBF is not constant: A multimodal 9.4T NIRS-MRI study on animal model1Biomedical Engineering Graduate Program, University of Calgary, Calgary, AB, Canada, 2Department of Radiology, University of Calgary, Calgary, AB, Canada, 3Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada, 4Experimental Imaging Centre, University of Calgary, Calgary, AB, Canada
Synopsis
Non-invasive imaging of cerebral oxygen delivery and consumption is crucial to understand neurovascular coupling and oxidative metabolism. We combine NIRS and MRI to determine the correlation between mitochondrial status and perfusion in the cortex of mice, when exposed to hypercapnia or varying oxygen levels. There was no correlation between perfusion and redox state under hypercapnia, while a strong correlation was observed under varying oxygen levels. This proves that the relationship between perfusion and mitochondrial redox can be quantified non-invasively in vivo. Such simultaneous measurements, in neurovascular diseases, will help determine whether there is abnormal neurovascular coupling or abnormal oxidative metabolism.
INTRODUCTION
The coupling of cerebral blood flow (CBF) and metabolic rate of oxygen, i.e., neurovascular coupling, is an important parameter in studies of neurological disorders. When neurovascular coupling is normal, it is assumed that there is a constant relationship between CBF and metabolic rate. Under these conditions, the redox state of the mitochondrial enzyme cytochrome c oxidase (CCO), which is directly responsible for oxygen consumption in the cell, might change in proportion to CBF. Alterations in this relationship could indicate an abnormal or diseased condition.To determine whether this relationship is constant, or whether it depends on the stimulus that affects CBF, we applied two challenges - hypercapnia, and varying levels of inspired oxygen. We used a novel multimodal NIRS-MRI system in mouse models to determine if the stimulus used to manipulate CBF, impacts the correlation between CCO redox and CBF.
METHODS
A 9.4T Bruker MRI with a 35 mm volume coil was used to non-invasively quantify CBF with an arterial spin labeling (ASL) sequence; A single axial slice was acquired around the bregma using a CASL-HASTE sequence with the following parameters: TR/TE=3000/13.3 ms, matrix size = 128x128 pixels, FOV = 25.6x25.6 mm, slice thickness = 1.5 mm, 16 averages. In total, 4 perfusion images were collected per measurement: 2 controls and 2 tagged images, to correct for magnetization transfer. Following these, a T1 map was obtained in the same location using a RARE-VTR sequence where TE = 20 ms, TR=100, 500, 1000, 3000 and 7500 ms. The 4 perfusion images and T1 map are collected over a period of 14 min. CBF (perfusion) was calculated from a region of interest in the cortex1.We measured the concentration and redox state of CCO using a custom-built broadband NIRS device and in-house developed processing algorithm to quantify oxidized and reduced CCO.
Fourteen C57Bl6 mice were anesthetized and spontaneously ventilated (30% O2, 70% N2), and physiologically stabilized prior to data acquisition.
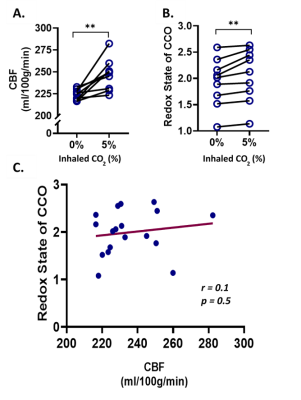
To characterize the effect of inhaled CO2, nine mice were monitored using the multimodal NIRS-MRI, before and during the induction of hypercapnia (5% CO2).
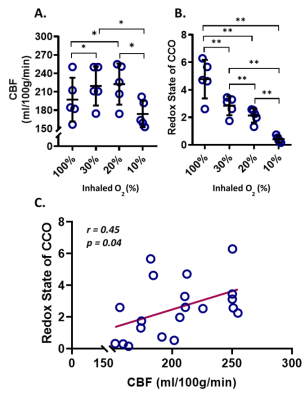
To study the response of CBF and CCO when an external oxygen challenge is imposed, five mice were monitored under 4 different levels of inhaled O2, balanced with N2: normoxia (FiO2 = 30%), hyperoxia (FiO2 = 100%), mild hypoxia (FiO2 = 20%), and severe hypoxia (FiO2 = 10%).
RESULTS
During the hypercapnia challenge, there was a significant increase in the redox state of CCO (+ 6.7%, p = 0.006), and CBF (+ 10%, p = 0.004). However, there was no significant correlation between cortical CBF and CCO redox state (r = 0.1, p = 0.5).Under hyperoxia, CBF decreased (- 10.1%, p = 0.03), while the redox state of CCO increased significantly (+ 68.3%, p = 0.005), relatively to normoxia. Under mild hypoxia there was no significant change in CBF, while under severe hypoxia, CBF decreased significantly (- 20.7%, p = 0.03). The redox state of CCO decreased significantly both under mild (- 25.0%, p = 0.001) and severe hypoxia (- 85.6%, p = 0.001), compared to normoxia. There was a minor but significant positive correlation (r = 0.45, p = 0.04) between redox state and CBF over the whole oxygenation range.DISCUSSION
Hypercapnia resulted in vasodilation and increased CBF. Hyperoxia resulted in vasoconstriction and reduced CBF. CBF changed in opposite directions while the redox state of CCO increased with both conditions. This implies that there are factors, other than oxygen availability that may affect the redox state of CCO and therefore the metabolic rate of oxygen. For instance, hypercapnia is likely to lead to higher levels of oxidized CCO through the mechanism involving reduced intracellular pH, and through the removal of the electron transport chain reducing agents in earlier stages of the cellular respiration2. Moreover, hypercapnia increases Nitric Oxide (NO) production3 which is known to influence CBF and to be a competitive inhibitor of CCO, therefore it is responsible for reducing the oxygen consumption by CCO and increasing the oxygen tension in the tissue4.CONCLUSION
Monitoring CCO in vivo simultaneously with CBF lays the groundwork for investigations of the mechanisms underlying neurovascular coupling. The association between CBF and the redox state of CCO is not fixed. Oxygen availability in the tissue is not the only factor driving the change in redox state of CCO and its association with CBF. The multimodal NIRS-MRI system allows us to study fundamental aspects of regulation of oxidative metabolism and will provide new insight into mitochondrial damage in neurological diseasesAcknowledgements
This work was supported by an NIH R21 grant, Canada Foundation for Innovation (CFI), Natural Sciences and Engineering Research Council (NSERC), Discovery grant, and the Biomedical Engineering Graduate program (BMEG) at U of C.
References
1. Buxton RB. Quantifying CBF with arterial spin labeling. Journal of magnetic resonance imaging: JMRI. Dec 2005;22(6):723-726.
2. Hempel FG, Jobsis FF, LaManna JL, Rosenthal MR, Saltzman HA. Oxidation of cerebral cytochrome aa3 by oxygen plus carbon dioxide at hyperbaric pressures. J Appl Physiol Respir Environ Exerc Physiol. Nov 1977;43(5):873-879.
3. Fathi AR, Yang C, Bakhtian KD, Qi M, Lonser RR, Pluta RM. Carbon dioxide influence on nitric oxide production in endothelial cells and astrocytes: cellular mechanisms. Brain Res. Apr 22 2011;1386:50-57.
4. Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett. Aug 7 1995;369(2-3):136-139.
Figures