0520
Longitudinal associations between blood biomarkers and white-matter MRI in sport-related concussion: A study of the NCAA-DoD CARE Consortium1Department of Radiology and Imaging Sciences, Department of RadioIndiana University School of Medicine, Indianapolis, IN, United States, 2Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indianapolis, IN, United States, 3National Institute of Health, Bethesda, MD, United States, 4Department of Radiology, Indiana School of Medicine, Indianapolis, IN, United States, 5Department of Biostatistics and Health Data Science, Indiana University School of Medicine, Indianapolis, IN, United States, 6Department of Neurosurgery, Medical College of Wisconsin, Milwaukee, WI, United States, 7Department of Epidemiology and Biostatistics, Indiana University School of Public Health, Bloomington, IN, United States, 8Department of Neurosurgery, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA, United States, 9Family Medicine, Ronald Reagan UCLA Medical Center, UCLA Health - Santa Monica Medical Center, Los Angeles, CA, United States, 10Department of Exercise and Sport Science, Matthew Gfeller Sport-Related Traumatic Brain Injury Research Center, Chapel Hill, NC, United States, 11School of Biomedical Engineering and Sciences, Wake-Forest and Virginia Tech University, Roanoke, VA, United States, 12School of Biomedical Engineering and Sciences, Wake-Forest and Virginia Tech University, Blacksburg, VA, United States, 13NeuroTrauma Research Laboratory, Michigan Concussion Center, University of Michigan, Ann Arbor, MI, United States, 14Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN, United States
Synopsis
White matter structural changes occur when an athlete experiences a concussion. To determine if serum biomarkers are a good concussion diagnosis, it seems prudent to associate white matter structural changes to longitudinal biomarker samples. Longitudinal blood biomarkers and DTI scanning from 77 collegiate athletes were collected across 3 timepoints and tested for associations. Tau was found to have the most significant associations. It was concluded that tau is the most sensitive for white-matter micro-structures.
Objective
To study longitudinal associations between blood-based neural biomarkers, including total tau, neurofilament light (NfL), glial fibrillary acidic protein (GFAP), and ubiquitin C-terminal hydrolase-L1 (UCH-L1) and white-matter neuroimaging biomarkers in concussed collegiate athletes during the acute to subacute phase of sport-related concussion (SRC).Methods
Clinical and imaging data of 77 collegiate athletes from the Concussion Assessment, Research and Education (CARE) Consortium were included in the analyses. Participants completed same-day clinical assessments, blood draws, and diffusion tensor imaging (DTI) at 3 time-points: 24-48 hours post-injury, asymptomatic state, and 7 days following return-to-play. Blood sample collection and biomarker analysis: The collection of blood samples and biomarker analysis followed the CARE protocol. Briefly, blood samples were collected by venipuncture with a 10-ml red-top tube prior to being centrifuged and aliquoted into cryovials. The cryovials were stored upright in a -80C freezer until analysis. The serum biomarker levels were analyzed using single molecular array technology (SimoaTM, Quanterix Corp., Lexington, MA) with a multi-plex technology that simultaneously quantified total-tau, NfL, GFAP, and UCH-L1. Diffusion imaging protocol: The neuroimaging imaging acquisition protocol and longitudinal MRI quality assurance/control followed the original CARE design. Briefly, diffusion MRI scans were performed on participants on Siemens MAGNETOM 3T Tim Trio or 3T Prisma scanners across the three ARC sites (VT, UNC, and UCLA). A single-shot echo-planar imaging sequence with a twice-refocused spin echo was used. The diffusion-encoding scheme consisted of 30 directions at b-value of 1000 s/mm2 and 8 b0 (b-value= 0 s/mm2). One of the b0 volumes was acquired with a reversed phase-encoding direction. Other MRI parameters were echo time (TE) = 98 ms, repetition time (TR) = 7900 ms, field-of-view (FOV) = 243 mm, matrix size = 90 x 90, whole brain coverage of 60 slices with a slice thickness of 2.7 mm, and isotropic resolution of 2.7 mm. DTI probabilistic tractography was performed for each subject at each time-point to render 27 subject-specific major white-matter tracts. The microstructural organization of these tracts was characterized by DTI metrics, including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD). Statistics: Mixed-effects models with random intercepts were applied to test whether white-matter microstructural abnormalities manifesting in DTI metrics are associated with the blood-based biomarkers at the same time-point. An interaction model was used to test if the association varies across time-points. A lagged model was used to test if early blood-based biomarkers predict later microstructural changes.Results
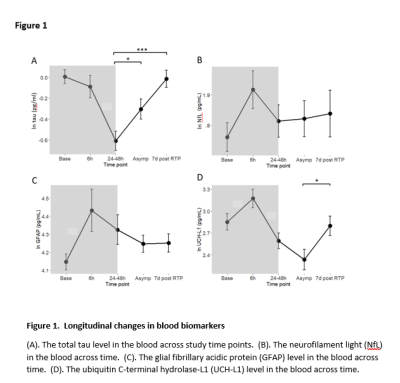
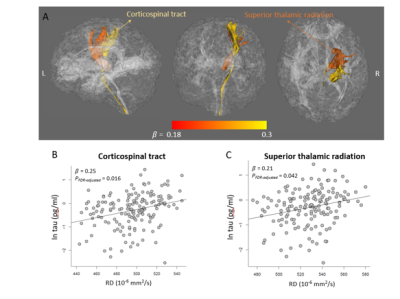
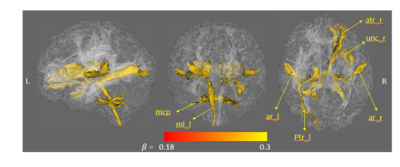
The characteristics of the participating athletes are listed in Table 1. The 77 concussed athletes were football (n=45), soccer (n=24), and lacrosse (n=8) players. Mean levels of the blood biomarkers (after natural-logarithm transform) at each time-point are reported at the bottom half of Table 1 and Figure 1. During the acute to subacute phase of SRC (white zone in Figure 1), most of the blood biomarkers continued recovering toward the baseline level at varying rates. While NfL and GFAP did not change over this period, tau and UCH-L1 exhibited significant longitudinal changes. Among the 4 blood biomarkers, total tau had the most significant same-time associations and lagged associations with the DTI metrics across the 27 white-matter tracts. In particular, tau was significantly positively associated with RD (pFDR-adjusted =0.016) and MD (pFDR-adjusted =0.068) in the right corticospinal tract and superior thalamic radiation (Figure 2). Also, early tau was significantly positively associated with later time-point RD (psFDR-adjusted <0.1) in 7 white-matter tracts (Figure 3). In the association analyses with the DTI metrics, NfL showed a significant association at the asymptomatic time-point (psFDR-adjusted <0.05) and GFAP showed a significant association at 7-days following return-to-play (psFDR-adjusted <0.05).Discussion
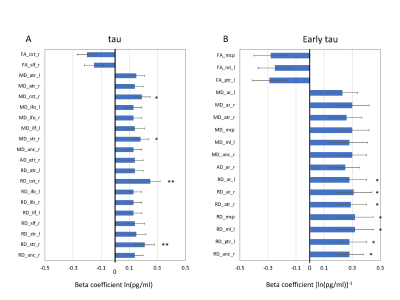
In these analyses, higher tau levels were significantly associated with higher radial diffusivity and mean diffusivity, as well as, to a minor extent, lower fractional anisotropy (Figure 4). Overall, the directions of changes in DTI-metrics describe consequences of degraded axons with increased organizational dispersion and increased water diffusion freedom perpendicular to the axons. The underlying pathophysiological explanation for increased radial diffusivity could be axonal beading, reduced axonal packing density, and/or compromised myelin sheaths. This observation complements our previous findings of significant group differences in the DTI mean and radial diffusivities between concussed and control athletes, as well as persistent elevation in the white matter of concussed athletes. Our results in humans are supported by a rat model of mTBI using mild fluid percussion injury, in which increased RD and AD, and decreased FA were observed in a similar post-injury time span. In the same rat model, the blood tau level decreased after brain injury, similar to the longitudinal changes in tau reported in the present human study.Conclusions
This prospective study using data from the CARE consortium demonstrated that in the acute to subacute period of SRC, white-matter microstructural integrity detected by DTI neuroimaging was associated with central nervous system-related metabolites in the blood. Total tau in the blood was the most sensitive biomarker for white-matter microstructures.Acknowledgements
This publication was made possible, in part, with support from the Grand Alliance Concussion Assessment, Research, and Education (CARE) Consortium, funded, in part by the National Collegiate Athletic Association (NCAA) and the Department of Defense (DOD) under Award NO W81XWH-14-2-0151. Other funding support includes National Institutes of Health grant R01 NS112303 to YCW and JH and R01 AG053993 to YCW.References
No reference found.Figures
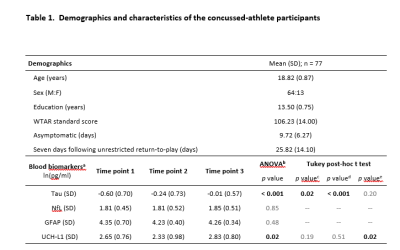
Table 1
a Logarithmically transformed for statistical tests.
b Analysis of variance (ANOVA) to test if there is any difference in blood biomarkers between time points.
c p-Values for post-hoc comparisons between timepoint 1 and timepoint 2;
d p-Values for post-hoc comparisons between timepoint 1 and timepoint 3;
e p-Values for post-hoc comparisons between timepoint 2 and timepoint 3.