0517
Automated Superstructure-based Segmentation of Ascending Arousal Network Nuclei for Diffusion Tractography1Center for Neurotechnology and Neurorecovery, Department of Neurology, Massachusetts General Hospital, Boston, MA, United States, 2Neurostatistics Research Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA, United States, 5Picower Institute, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States, 7Centre for Medical Image Computing, University College London, London, United Kingdom, 8Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Traumatic brain injury that causes sheering of white matter pathways that connect ascending arousal network (AAN) nuclei in the rostral brainstem to cortical and subcortical targets leads to disorders of consciousness. Connectivity studies involving these nuclei are limited due to their small size, vague boundaries, and lack of reliable annotations. We present a method to automatically segment AAN nuclei from diffusion MR volumes using image registration constrained by brainstem structures with definable contrast boundaries. We test AAN segmentation robustness with probabilistic tractography and provide new insights into connectivity between AAN nuclei and the thalamus.
Introduction
Severe traumatic brain injury (TBI) affects over 1 million people worldwide annually. Approximately 25% of patients with severe TBI die in the ICU1. Of those who survive, many suffer from prolonged cognitive deficits, and thousands never recover consciousness. The underlying pathology believed to cause TBI-related disorders of consciousness is white-matter shearing injury in the ascending arousal network (AAN)2.High Angular Resolution Diffusion Imaging (HARDI) has produced considerable advances in subcortical tractography3-5. There is still, however, limited attention to AAN connectivity. This is partly because brainstem-based AAN seed nuclei (AASN) are difficult to delineate due to their small size and lack of anatomical contrast in most MR sequences, implying that even manual annotation is problematic. This presents a two-fold challenge: 1) intensity-based segmentation of AASN becomes an ill-conditioned problem, and 2) direct validation of candidate segmentations becomes ambiguous due to unreliable gold-standard annotations. We propose a resolution-agnostic method to segment AASN in HARDI volumes by utilizing contrast-delineable “superstructure” regions of interest (ROIs) which spatially bound AASN to steer non-rigid registration of a multi-channel diffusion atlas to the HARDI volume. We then compare connectivity strengths between annotated and predicted AASN and the thalamus in ex-vivo volumes, followed by lower-resolution in-vivo volumes.
Methods
HARDI Acquisition/Preprocessing: Thirteen ex-vivo brain specimens were imaged on a 3T Siemens Trio. A diffusion-weighted SSFP sequence was used, with 750$$$\mu$$$m isotropic voxels, 90 diffusion-encoded directions (estimated b=4080s/m2) and 12 b0 volumes6. Temperature drift correction and eddy current correction was performed with FSL7.Superstructure Generation: Manually delineated Pons/Midbrain (PMB) superstructures in 8 ex-vivo volumes and 10 Human Connectome Project (HCP) in-vivo HARDI volumes8 (1.25mm isotropic voxels) were used to train a 3D U-Net9 to segment the PMB in two-channel fractional anisotropy and mean diffusivity (FAMD) volumes. We used aggressive data augmentation, which included resolution randomization, to account for inter-modal resolution/contrast differences10.
Registration: We back-registered an FAMD atlas11 containing gold-standard AASN labels12 onto a target FAMD volume. Our registration scheme is as follows:
1) Rigid + Affine registration of the whole atlas to the target volume.
2) Repeat Step 1 with PMB-masked volumes.
3) Non-rigid PMB-masked registration using basis-splines transformations penalized with "relaxed" diffusion regularization and compound Normalized Gradient Field13 (NGF) loss. Specifically: given the atlas PMB domain $$$ \mathcal{X} \subset \mathbb{R}^3 $$$, deformation field $$$ \mathcal{U}: \mathcal{X} \rightarrow \mathbb{R}^3 $$$, and $$$N$$$ superstructure masks $$$\textbf{M} = \{M_1,...,M_N \} $$$, our relaxed diffusion regularization is:
$$\mathfrak{R}_{relaxed} = \frac{\alpha}{2}\int_{\mathcal{X}}||\nabla \mathcal{U}(x)||_2^2 \times \text{smooth}_{\beta}\left\{\Psi_{\gamma_i,\delta_i,\epsilon_i}\left(\mathcal{D}(x,\textbf{M}) \right) \right\} dx$$
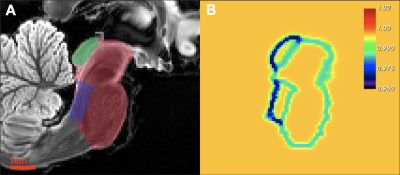
where $$$\mathcal{D}(x,M_i)$$$ is a distance transform from the $$$M_i$$$ boundary, $$$\Psi_{\gamma_i,\delta_i,\epsilon_i}(.)$$$ is the Gompertz function, and $$$\text{smooth}_{\beta}\{.\} $$$ is a smooth maximum over $$$\textbf{M}$$$. This relaxation permits greater deformation around superstructure contrast-boundaries while maintaining high regularity inside of the superstructure union under a low atlas-to-target contrast assumption14,15 (Figure 1).
For a single-channel PMB-masked NGF loss between the deformed atlas $$$\mathcal{T}\left(A_{PMB} \right)$$$ and target volume $$$T_{PMB}$$$ as $$$\mathcal{N}\left\{\mathcal{T}\left(A_{PMB}\right),T_{PMB} \right\} $$$, the compound loss, which facilitates superstructure overlap, is:
$$L_{total} = \alpha_0 \times \mathcal{N}\left\{\mathcal{T}\left(A_{PMB}\right),T_{PMB} \right\} + \sum_{i=1}^{N} \alpha_i \times \text{MSE}\left\{\mathcal{T}\left(M_i^{\text{atlas}}\right),M_i^{\text{target}}\right\} $$
where $$$\text{MSE}\{.,.\}$$$ is the mean squared error.
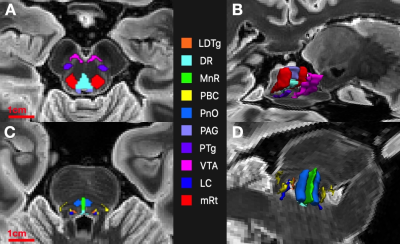
4) Propagated AASN-atlas labels serve as segmentations (Figure 2). We will henceforth refer to this segmentation scheme as Constrained Registration-Based SEGmentation (CRSEG).
Tractography: We performed probabilistic tractography with probtrackx16. We quantified connection strengths with a connectivity-probability (CP) metric17. Step-lengths for tract growing were set to half of the voxel size to account for resolution differences.
Results
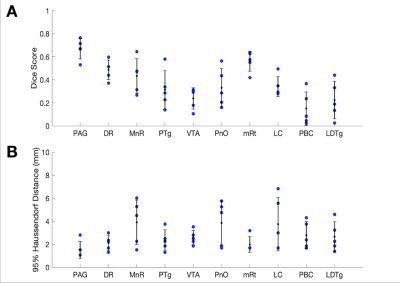
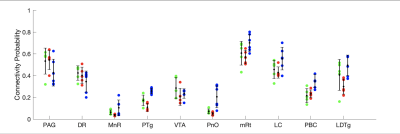
Sorensen-Dice coefficients and Hausdorff distances for 5 annotated ex-vivo specimens not used for U-net PMB training are provided for comparison of CRSEG predictions and corresponding expert-annotated AASN (Figure 3). To assess overall CP variability between CRSEG predictions and annotations, we performed probabilistic tractography between AASN and the thalamus on the annotated ex-vivo cases and 5 HCP in-vivo cases (Figure 4). Intra-subject CP MSE between ex-vivo annotations and CRSEG predictions are reported in Table 1.Discussion/Conclusion
We present CRSEG, a joint machine learning and registration-based scheme for AASN segmentation. We validate CRSEG robustness via comparisons with manual labelling and probabilistic tractography. Finally, we reveal consistent connectivity differences between AASN and the thalamus.Dice scores for AASN with some definable contrasts are higher (PAG average Dice=0.67, mRt average Dice=0.56). Volumes for AASN with lower Dice scores range from LDTg=57 to VTA=641 voxels. This, along with single-voxel thicknesses for some AASN, implies that any minor spatial shifts drastically affect Dice scores. Haussdorf distances, however, remain low, indicating accurate CRSEG localization. Furthermore, CP values indicate high coherence; CP MSE (except for LDTg (MSE=0.024)) all fall below 0.010. Moreover, similar in-vivo results reveal novel CP signatures between AASN and the thalamus (Figure 4). One limitation of this study is the small sample-size, which is due to the time-intensive labelling process for high resolution ex-vivo cases (average time for one reviewer is 5 hours). Another limitation is that CRSEG performance does not directly depend on registration and/or superstructure segmentation quality. CRSEG assumes that low-contrast AASN are in a standard anatomical configuration with typical morphology. In future work we aim to incorporate this variation from histological sections as spatial priors with the goal of Bayesian seed-smoothing for tractography. These methods have the potential to advance knowledge about brainstem connectivity in human consciousness and its disorders.
Acknowledgements
Research reported in this abstract was supported by National Institute of Biomedical Imaging and Bioengineering (NIBIB) of the National Institutes of Health under award number 5T32EB001680-17, the National Institute of Health (NIH) under award numbers 1R01AG070988-01 and 1RF1MH123195-0, Alzheimer’s Research UK with interdisciplinary grant number ARUK-IRG2019A-003, and the European Research Council (ERC) with starting Grant number 677697.
Data were provided in part by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
References
1. D. Jochems, K. van Wessem, R. Houwert, H. Brouwers, J. Dankbaar, M. van Es, M. Geurts, A. Slooter and L. Leenen, "Outcome in Patients with Isolated Moderate to Severe Traumatic Brain Injury," Crit Care Res Pract, 2018.
2. B. L. Edlow, R. L. Haynes, E. Takahashi,, J. P. Klein, P. Cummings, T. Benner, D. M. Greer, S. M. Greenberg, O. Wu, H. C. Kinney and R. D. Folkerth, "Disconnection of the ascending arousal system in traumatic coma," J Neuropathol Exp Neurol, vol. 72, no. 6, pp. 505-23, 2013.
3. D. Tuch , "Q-ball imaging," Magn Reson Med, vol. 52, p. 1358–72, 2004.
4. D. S. Tuch, T. G. Reese, M. R. Wiegell, N. Makris, J. W. Belliveau and V. J. Wedeen, "High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity," Magn Reson Med , vol. 48, no. 4, pp. 577-82, 2002.
5. J. Tournier, C. Yeh, F. Calamante, K. Cho, A. Connelly and C. Lin, "Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data," NeuroImage, vol. 42, no. 2, pp. 617-25, 2008.
6. G. Yang and J. A. McNab, "Eddy current nulled constrained optimization of isotropic diffusion encoding gradient waveforms," Magn Reson Med, vol. 81, no. 3, pp. 1818-1832, 2019.
7. S. Jbabdi, S. Sotiropoulos, A. Savio, M. Grana and . T. Behrens, "Model-based analysis of multishell diffusion MR data for tractography: How to get over fitting problems," Magn Reson Med, 2012.
8. D. Van Essen, S. Smith, D. Barch, T. Behrens, E. Yacoub, K. Ugurbil and for the WU-Minn HCP Consortium, "The WU-Minn Human Connectome Project: An overview," NeuroImage, vol. 80, no. 2013, pp. 62-79, 2013.
9. A. Wolny, L. Cerrone, A. Vijayan, R. Tofanelli, A. Barro, M. Louveaux, C. Wenzl, S. Strauss, D. Wilson-Sánchez, R. Lymbouridou, S. Steigleder, C. Pape, A. Bailoni, S. Duran-Nebreda, G. Bassel, J. Lohmann, M. Tsiantis, F. Hamprechet, K. Schneitz and Ma, "Accurate and versatile 3D segmentation of plant tissues at cellular resolution," Elife, vol. 9, 2020.
10. B. Billot, D. N. Greve, O. Puonti, A. Thielscher, K. Van Leemput, B. Fischl, A. V. Dalca and J. E. Iglesias, "SynthSeg: Domain Randomisation for Segmentation of Brain MRI Scans of any Contrast and Resolution," 2021. [Online]. Available: https://arxiv.org/abs/2107.09559.
11. F. Yeh, S. Panesar, D. Fernandes, A. Meola, M. Yoshino, J. Fernandez-Miranda, J. Vettel and T. Verstynen, "Population-averaged atlas of the macroscale human structural connectome and its network topology," NeuroImage, vol. 178, pp. 57-68, 2018.
12. B. Edlow , E. Takahashi , O. Wu, T. Benner , G. Dai , L. Bu, P. Grant , D. Greer, S. Greenberg, H. Kinney and R. Folkerth, "Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders," J Neuropathol Exp Neurol, vol. 71, no. 6, pp. 531-46, 2012.
13. E. Haber and J. Modersitzki, "Intensity Gradient Based Registration and Fusion of Multi-modal Images," MICCAI, pp. 726-733, 2006.
14. R. Stefanescu, X. Pennec and N. Ayache, "Grid powered nonlinear image registration with locally adaptive regularization," Medical Image Analysis, vol. 8, no. 3, pp. 325-342, 2004.
15. P. Thompson, R. Woods, M. Mega and A. Toga, "Mathematical/computational challenges in creating deformable and probabilistic atlases of the human brain," Hum Brain Mapp, vol. 9, no. 2, pp. 81-92, 2000.
16. T. Behrens, M. Woolrich, M. Jenkinson, H. Johansen-Berg, R. Nunes, S. Clare, P. Matthews, J. Brady and S. Smith, "Characterization and propagation of uncertainty in diffusion-weighted MR imaging," Magn Reson Med, vol. 50, no. 5, pp. 1077-1088, 2003.
17. S. Snider, Y. Bodien, M. Bianciardi, E. Brown, O. Wu and B. Edlow, "Disruption of the ascending arousal network in acute traumatic disorders of consciousness," Neurology , vol. 93, no. 13, pp. 1281-1287, 2019.
Figures