0508
Prepolarized MRI of Hard Tissues and Solid-State Matter1Tesoro Imaging S.L., Valencia, Spain, 2MRI-lab, Institute for Molecular Imaging and Instrumentation (i3M), Spanish National Research Council (CSIC) and Universitat Politècnica de València (UPV), Valencia, Spain
Synopsis
Prepolarized Magnetic Resonance Imaging is a long established technique conceived to counteract the loss in signal strength inherent to low field MRI systems. When it comes to hard biological tissues and solid state matter, PMRI is severely restricted by their ultra short characteristic relaxation times. Here we demonstrate that hard tissue prepolarization is possible with a 0.26 T scanner designed for dental MRI. These results can be applied to clinical dental imaging, making low field PMRI scanners a possible replacement for hazardous X ray systems.
Introduction
Low-Field Magnetic Resonance Imaging (LF-MRI) is gaining momentum as an affordable alternative to clinical MRI, currently expensive and inaccessible [1-2]. Lowering the field strength can drastically reduce the economic and energetic requirements, but the signal-to-noise ratio (SNR) of the magnetic resonance signals is also compromised. Prepolarization is a technique designed to partially compensate for the SNR loss in LF-MRI [3-4], and could be of special relevance for hard biological tissues, where hydrogen content is sparse and signals decay very fast [5-6]. In Prepolarized MRI (PMRI), the Boltzmann magnetization of the sample is boosted by an intense magnetic pulse of amplitude 𝐵p before the start of the imaging pulse sequence, which is then executed at a lower homogeneous 𝐵0. For efficient PMRI, the prepolarization pulse must be turned off in a time 𝑡off much shorter than the sample 𝑇1 relaxation time over which the extra magnetization is lost. This is easily met for soft tissues, but for solid-state matter and hard tissues, which feature short 𝑇1 times, prepolarization is much more challenging. Here we demonstrate that efficient hard tissue prepolarization and imaging is within reach with a special-purpose 0.26 T MRI scanner equipped with suitable high-power electronics [7].Apparatus
As a result of the short 𝑇1 timescales of solids, hard tissue prepolarization poses a significant engineering challenge to achieve fast enough tdel1 times (see Figure 1(a)). This delay is the time from the moment the prepolarization pulse starts to be switched off until the beginning of the radio-frequency pulse, to remove sufficient residual magnetic energy from the prepolarizer. Our group has designed and built a prepolarizer coil whose main parameters are 𝐿 ≈ 600 μH, 𝑅 ≈ 75 mΩ and 𝜂 ≈ 1.9 mT/A [6]. The high power electronics setup for the prepolarizer module are based on a TTL signal that is amplified in two stages, first in a homemade variable-gain low-voltage amplifier, and then in a high power (400 A and 750 V) gradient amplifier from International Electric Co. (GPA 400-750). The latter can ramp currents from 0 to ±260 A in 200 μs in the load.Experimental results
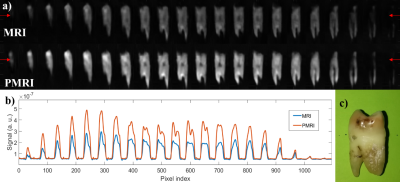
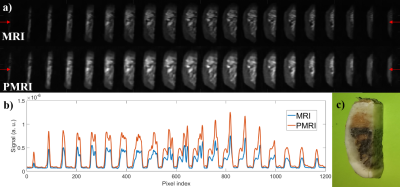
For a first test, we employed a sample of photopolymer resin [8], which features relaxation parameters comparable to the enamel (𝑇1 ≈ 23.1 ms and 𝑇2 ≈ 650 μs). To determine the SNR enhancement, we apply a prepolarization pulse of length 𝑡p = 160 ms, long enough to reach saturation. Next, a resonant 𝜋/2 RF pulse rotates the magnetization to the transverse plane. Both pulses are separated by a wait time 𝑡del1 = 3 ms, long enough to avoid Larmor frequency shifts and distortions in the Free Induction Decay (FID). Acquisition starts 100 μs after the RF pulse and the resulting FIDs (see Figure 1) are acquired for 𝑡acq = 2 ms. This protocol is repeated for 𝐵t ≈ 0.33, 0.39, 0.47 and 0.56 T, leading to experimental (theoretical) SNR gains of 1.24 (1.24), 1.43 (1.44), 1.71 (1.72) and 1.964 (1.98). The observed deviations could arise from: i) mechanical vibrations due to magnetic forces, ii) induced Eddy currents or iii) off-resonant spin evolution due to a time-dependent Larmor frequency. All three are more pronounced for intense 𝐵p values and short 𝑡del1 times.To demonstrate the system’s capability for PMRI of hard biological tissues, we employ a PETRA (Pointwise Encoding Time-reduction with Radial Acquisition, [9]) with a prepolarization stage before each RF excitation. Figures 2 and 3 show comparative MRI/PMRI images of a human molar tooth (𝑇1 ≈ 20.3 ms) and cattle rib bone (𝑇1 ≈ 19.3 ms). TR and NEX are fixed to 250 ms and 12 for tooth images and 280 ms and 11 for bone images. The bottom rows in the figures correspond to scans in which a prepolarization pulse is triggered with a current intensity of ≈260 A (𝐵t ≈ 0.56 T), during 𝑡p = 90 ms. The pulse sequence for the top rows are identical, but the prepolarization pulse is not triggered (𝐵p = 0, 𝐵t ≈ 0.26 T). To quantify the influence of prepolarization, Figure 2(b) and Figure 3(b) show the same profile along a horizontal line of the images in (a). The experimental (theoretical) SNR gain is 1.97 (2.02) for the tooth and 1.99 (2.00) for the cattle rib.
Conclusions
We have shown that it is possible to enhance the quality of MRI of hard tissues at low magnetic fields by means of a high power prepolarizer module, for a total cost of ≈ 20 k€. The results in this work are of potential application to clinical dental MRI. This would require a prepolarizer magnet large enough to fit a human head. Our estimations suggest this is realistic with 𝐵p ≈ 0.3 T at 210 A and times of switching around 7 ms.Acknowledgements
This work was supported by the Ministerio de Ciencia e Innovación of Spain through research grant PID2019-111436RBC21. Action co-financed by the European Union through the Programa Operativo del Fondo Europeo de Desarrollo Regional (FEDER) of the Comunitat Valenciana 2014-2020 (IDIFEDER/2018/022). JMG and JB acknowledge support from the Innodocto program of the Agencia Valenciana de la Innovación (INNTA3/2020/22 and INNTA3/2021/17).References
[1] J. P. Marques, F. F. Simonis, and A. G. Webb, “Low-field MRI: An MR physics perspective,” Journal of Magnetic Resonance Imaging, vol. 49, no. 6, pp. 1528–1542, jun 2019. [Online]. Available: https://onlinelibrary.wiley.com/doi/abs/10.1002/jmri.26637
[2] M. Sarracanie and N. Salameh, “Low-Field MRI: How Low Can We Go? A Fresh View on an Old Debate,” Frontiers in Physics, vol. 8, p. 172, jun 2020. [Online]. Available: https: //www.frontiersin.org/article/10.3389/fphy.2020.00172/full
[3] P. Morgan, S. Conolly, G. Scott, and A. Macovski, “A readout magnet for prepolarized MRI,” Magnetic Resonance in Medicine, vol. 36, no. 4, pp. 527–536, oct 1996. [Online]. Available: http://doi.wiley.com/10.1002/mrm.1910360405
[4] C. Kegler, H. Seton, and J. Hutchison, “Prepolarized fast spin-echo pulse sequence for low-field MRI,” Magnetic Resonance in Medicine, vol. 57, no. 6, pp. 1180–1184, jun 2007. [Online]. Available: http://doi.wiley.com/10.1002/mrm.21238
[5] J. M. Algarín, E. Díaz-Caballero, J. Borreguero, F. Galve, D. Grau-Ruiz, J. P. Rigla, R. Bosch, J. M. González, E. Pallás, M. Corberán, C. Gramage, S. Aja-Fernández, A. Ríos, J. M. Benlloch, and J. Alonso, “Simultaneous imaging of hard and soft biological tissues in a low-field dental MRI scanner,” Scientific Reports, vol. 10, no. 1, p. 21470, 2020. [Online]. Available: https://doi.org/10.1038/s41598-020-78456-2
[6] J. P. Rigla, J. Borreguero, C. Gramage, E. Pallas, J. M. Gonzalez, R. Bosch, J. M. Algarin, J. V. Sanchez-Andres, F. Galve, D. Grau- Ruiz, R. Pellicer, A. Rios, J. M. Benlloch, and J. Alonso, “A Fast 0.5 T Prepolarizer Module for Preclinical Magnetic Resonance Imaging,” IEEE Transactions on Magnetics, 2021.
[7] J. M. González, J. Borreguero, E. Pallás, J. P. Rigla, J. M. Algarín, R. Bosch, F. Galve, D. Grau-Ruíz, R. Pellicer, A. Ríos, J. M. Benlloch and J. Alonso.“Prepolarized MRI of Hard Tissues and Solid-State Matter.” (2021) (arxiv.org ID 2110.03417)
[8] R. Rai, D. Manton, M. Jameson, S. Josan, M. Barton, L. Holloway, and G. Liney, “3d printed phantoms mimicking cortical bone for the assessment of ultrashort echo time magnetic resonance imaging,” Medical Physics, vol. 45, 12 2017.
[9] D. M. Grodzki, P. M. Jakob, and B. Heismann, “Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA),” Magnetic Resonance in Medicine, vol. 67, no. 2, pp. 510–518, feb 2012.
Figures