0497
The spatiotemporal organization of cortical microstructural development in children and adolescents with diffusion MRI1USC Mark and Mary Stevens Institute for Neuroimaging and Informatics, University of Southern California, Los Angeles, CA, United States
Synopsis
Neocortical maturation is a dynamic process that proceeds in a hierarchical manner; however, the spatiotemporal organization of cortical microstructure with diffusion MRI has yet to be fully defined. This study characterized cortical microstructural maturation using fwe-DTI and NODDI in a cohort of 637 children and adolescents between 8 and 21 years of age. We found heterogeneous developmental patterns broadly demarcated into frontal, temporal and occipitoparietal domains where NODDI metrics increased and fwe-DTI metrics decreased with age. Our findings corroborate previous histological and neuroimaging studies that show spatially-varying patterns of cortical maturation that may reflect unique developmental processes of cytoarchitectonically-determined regions.
Introduction
The neocortex undergoes a protracted period of maturation that continues into adolescence and proceeds in a hierarchical manner where primary sensorimotor regions develop earlier than association regions. Because the neocortex is spatially organized according to cytoarchitectonically determined regions, understanding the developmental dynamics of cortical microstructure can provide insight into the maturation and refinement of the cellular properties that define these regions. Heterogeneous patterns of cortical microstructural development has previously been characterized in children using T1w/T2w contrasts [1] and magnetization transfer [2]. While these contrasts provide insight into cortical myelination, they lack sensitivity to the wide variety of cellular features that occupy the cortex. Diffusion MRI uses multiple microstructural models sensitized to different tissue properties and can provide complementary insight into cortical cytoarchitectonics. Previous studies have characterized the spatial organization of global cortical microstructure with dMRI in adults [3], [4] and neonates [5], [6]; however, the developmental trajectory of cortical microstructure in children and adolescents has yet to be elucidated. The goal of the present study is to characterize the spatiotemporal development of cortical microstructural maturation in a large cohort of typically developing children and adolescents between 8 and 21 years of age using quantitative diffusion MRI models, free water-eliminated diffusion tensor imaging (fwe-DTI) [7] and neurite orientation dispersion and density imaging (NODDI) [8].Methods
Multi-shell diffusion MRI (b=1500 s/mm2, 3000 s/mm2, 92-93 diffusion-encoding directions per shell, 1.5 mm isotropic voxel, TR=3.23 s) and T1w images (8 mm isotropic voxels, FOV = 256 x 240 x 166 mm, matrix = 320 x 300 x 208 slices, TR = 2500 ms, TI = 1000 ms, TE = 1.8/3.6/5.4/7.2 ms, FA = 8 degrees) were acquired from 637 typically developing children and adolescents between 8 and 21 years of age (13.8±3.8 years, 323 F) in the Lifespan Human Connectome Project-Developing (HCP-D) cohort [9]. MRI data was processed with the HCP minimal preprocessing pipeline [10]. The parameters AD, RD, MD and FA were derived from fwe-DTI using iterative least squares optimization [11] and the NODDI parameters FICVF, FISO and ODI were calculated using the spherical mean technique implemented with the QIT [12]. Pial and white matter surfaces were extracted using Freesurfer 6 (http://surfer.nmr.mgh.harvard.edu/) and dMRI maps were aligned to T1w native space using a rigid body transformation. Microstructural surface mapping was performed using a weighted average along the shortest path between each pial and white matter surface vertices, with the strongest weight assigned to the centroid of the path [13]. Laplacian smoothing was then carried out with 30 iterations of lambda=.8. Cortical surfaces and their features were then registered to an average template to ensure one-to-one correspondence among subjects. The main effect of age on diffusion parameters after controlling for the effects of scanner site and sex was carried out at each vertex using a general linear model with random field theory multiple comparison correction.Results
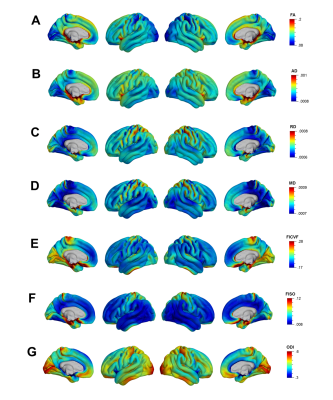
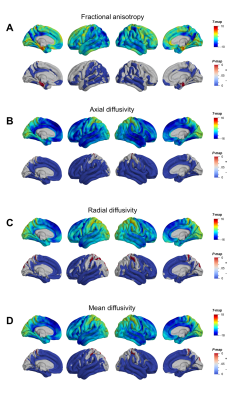
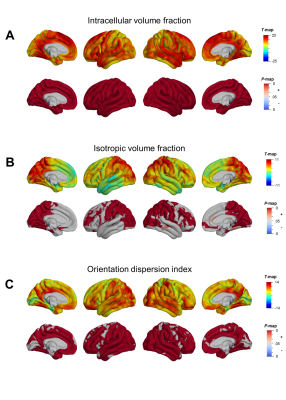
Mean cortical DTI and NODDI maps for the cohort are shown in Figure 1. Heterogeneous patterns of cortical microstructural maturation were observed across diffusion metrics bilaterally. In general, we found DTI metrics decreased (Figure 2) and NODDI metrics increased with age (Figure 3). Age-related FA decreases were predominantly localized to temporal, parietal and lateral occipital cortices (Figure 2A), while AD, RD and MD decreased in frontal and temporal regions and increased in the postcentral gyrus (Figure 2B-D). Cortical FICVF and ODI age-related increases were observed globally (Figure 3A,C), while FISO age effects occurred primarily in occipital and temporal regions (Figure 3B).Discussion
In the present study, we observed heterogeneous spatial patterns of cortical maturation using diffusion MRI metrics sensitized to different microstructural features. Global age-related increases in cortical neurite density index and orientation dispersion indicating increased intracellular fluid flow are consistent with previous histological studies that show child and adolescent development is characterized by cortical myelination and dendritic arborization [13]. Reductions in DTI metrics in the frontal and temporal lobes suggest age-related increases in cortical tissue density, while increased FISO, AD, RD and MD and decreased FA in occipital and parietal regions indicate elevated cortical free water with age. This latter finding within the occipitoparietal regions corresponds to primary sensory, auditory and visual areas and may reflect developmental pruning processes, which are known to occur earlier in evolutionarily-conserved sensorimotor regions compared to higher order association regions [14]. Together, our findings generally recapitulate previous research that demonstrates the spatial pattern of cortical maturation coincides with hierarchical functional and cytoarchitectonic gradients.Conclusion
The results from this study sheds new light on the maturation of cortical microstructure and demonstrates the utility of diffusion metrics to study the cytoarchitectural organization of the cortex. Future studies will aim to corroborate our findings with histologically matched templates to better understand how microstructural metrics derived from non-invasive neuroimaging techniques reflect the developmental processes that give rise to cognitive functions and complex behaviors.Acknowledgements
The image computing resources provided by the Laboratory of Neuro Imaging Resource (LONIR) at USC are supported in part by National Institutes of Health (grant number P41EB015922). Author RPC is supported in part by grant number 2020-225670 from the Chan Zuckerberg Initiative DAF, an advised fund of Silicon Valley Community Foundation. Author KML is supported by the National Institutes of Health (NIH) Institutional Training Grant T32AG058507. Data collection and sharing for this project was funded by the Human Connectome Project.References
[1] L. B. Norbom et al., “Maturation of cortical microstructure and cognitive development in childhood and adolescence: A T1w/T2w ratio MRI study,” Hum. Brain Mapp., vol. 41, no. 16, pp. 4676–4690, 2020.
[2] N. M. Corrigan et al., “Myelin development in cerebral gray and white matter during adolescence and late childhood,” Neuroimage, vol. 227, no. December 2020, 2021.
[3] H. Fukutomi et al., “Neurite imaging reveals microstructural variations in human cerebral cortical gray matter,” Neuroimage, vol. 182, no. May 2017, pp. 488–499, 2018.
[4] J. Schmitz et al., “Hemispheric asymmetries in cortical gray matter microstructure identified by neurite orientation dispersion and density imaging,” Neuroimage, vol. 189, no. January, pp. 667–675, 2019.
[5] M. Ouyang et al., “Differential cortical microstructural maturation in the preterm human brain with diffusion kurtosis and tensor imaging,” Proc. Natl. Acad. Sci. U. S. A., vol. 116, no. 10, pp. 4681–4688, 2019.
[6] D. Batalle et al., “Different patterns of cortical maturation before and after 38 weeks gestational age demonstrated by diffusion MRI in vivo,” Neuroimage, vol. 185, no. April 2018, pp. 764–775, 2019.
[7] O. Pasternak, N. Sochen, Y. Gur, N. Intrator, and Y. Assaf, “Free water elimination and mapping from diffusion MRI,” Magn. Reson. Med., vol. 62, no. 3, pp. 717–730, 2009.
[8] H. Zhang, T. Schneider, C. A. Wheeler-Kingshott, and D. C. Alexander, “NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain.,” Neuroimage, vol. 61, no. 4, pp. 1000–16, Jul. 2012.
[9] M. Harms et al., “Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects,” Neuroimage, vol. 183, pp. 972–984, 2018.
[10] M. F. Glasser et al., “The minimal preprocessing pipelines for the Human Connectome Project,” Neuroimage, vol. 80, pp. 105–124, 2013.
[11] A. R. Hoy, C. G. Koay, S. R. Kecskemeti, and A. L. Alexander, “Optimization of a free water elimination two-compartment model for diffusion tensor imaging,” Neuroimaging, vol. 103, pp. 323–333, 2014.
[12] R. P. Cabeen, D. H. Laidlaw, and A. W. Toga, “Quantitative Imaging Toolkit : Software for Interactive 3D Visualization , Data Exploration , and Computational Analysis of Neuroimaging Datasets,” in ISMRM-ESMRMB Abstracts, 2018, pp. 12–14.
[13] R. P. Cabeen, A. W. Toga, and J. M. Allman, “Frontoinsular cortical microstructure is linked to life satisfaction in young adulthood,” Brain Imaging Behav., 2021.
[14] Y. Patel, J. Shin, P. A. Gowland, Z. Pausova, and T. Paus, “Maturation of the Human Cerebral Cortex during Adolescence: Myelin or Dendritic Arbor?,” Cereb. Cortex, vol. 29, no. 8, pp. 3351–3362, 2019.
[15] V. J. Sydnor et al., “Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology,” Neuron, vol. 109, no. 18, pp. 2820–2846, 2021.
Figures