0496
Ablative Efficacy of MR-guided Focused Ultrasound in Pediatric Patients: A Retrospective Study1Radiology, Stanford University, Palo Alto, CA, United States
Synopsis
Performance of MRgFUS in pediatric patients has not been well characterized. Retrospective review of 21 MRgFUS treatments in 9 patients below the age of 18 shows significant reduction in viable tumor volume in a variety of indications including desmoid tumors, vascular malformations, and osteoid osteoma. MRgFUS is an effective ablative therapy in children that warrants further investigation.
Introduction
Magnetic Resonance guided Focused Ultrasound (MRgFUS), also known as high-intensity focused ultrasound (HIFU) is a minimally invasive technique that provides targeted thermal ablation of tissue. MRgFUS has many applications in adults, including in the brain, uterus, prostate, and bone [1]. Ablation is performed using ultrasound energy that can be directed to a focus inside the body, resulting in a small ablation zone with sparing of surrounding tissue; MRI guidance provides precise targeting, delineates critical anatomy to avoid, and can be used to monitor tissue temperature. Given its lack of ionizing radiation and precise ablation zones, MRgFUS has many qualities that are appealing in the pediatric setting. However, only limited studies in pediatric patients have been performed [2] and the efficacy of ablation in pediatric patients has not been characterized.Methods
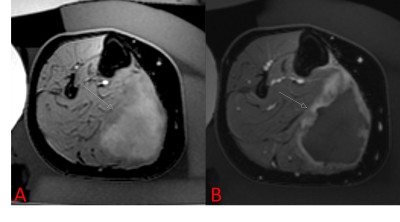
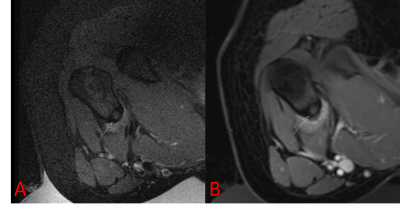
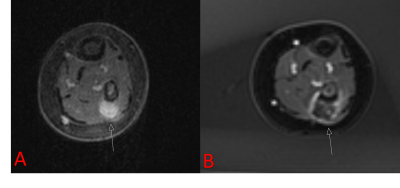
Patient’s guardians provided informed consent before treatment. When applicable, patient assent was obtained. Patients who had undergone MRgFUS were retrospectively identified in this IRB-approved, HIPAA compliant study; patients were included if they were treated between 2013 and 2020 and were less than 18 years old on the day of treatment. All treatments were performed using an ExAblate 2100 MRgFUS system with an in-table 1 MHz transducer (InSightec, Tirat-Carmel, Israel) installed with a 3T MR system (Discovery 750w, GE Healthcare, Waukesha, Wisconsin). Treatment in pediatric patients constitutes off-label use of the technology. Patients were treated for a variety of indications, all under general anesthesia (Figure 1). Pre-procedure imaging with or without gadolinium-containing intravenous contrast was performed. After ablation, contrast-enhanced imaging was performed.Retrospectively, total lesion volumes were segmented before and after treatment. The entirety of the lesion was assumed to be perfused prior to treatment. In some cases, the entirety of the lesion was not targeted; in these instances, the targeted lesion volume was also recorded. Non-perfused volume after ablation was determined based on non-enhancing tissue. Lesion volumes were manually segmented by contouring the lesion area on each imaging slice (Horos, Nimble Co LLC, Annapolis, Maryland). For patients who expressed pain related to their lesion, Numerical Rating Scale (NRS) pain scores were recorded before treatment and in follow-up. Number of sonications, median sonication energy, and treatment-related adverse events were also recorded.
Changes in viable lesion volume before and after MRgFUS were tested by determining if the viable post-ablation lesion volume had decreased by more than 50% (i.e. 50% efficacy threshold). Statistical analysis was performed in RStudio (RStudio PBC, Boston, MA) with an exact one-sided Wilcoxon test of the median percent reduction against a null hypothesis of 50% or less reduction in volume. Difference was considered statistically significant at p < 0.05.
Results
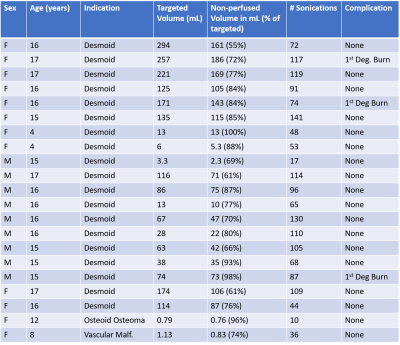
9 patients under the age of 18 underwent MRgFUS between 2013 and 2018, with a total of 21 treatments as some patients were treated more than once. The median age of patients was 14 years (range 3-17 years). The most common indication was desmoid fibromatosis (n = 19), although osteoid osteoma (n = 1) and vascular malformation (n = 1) were also treated. The median targeted lesion volume for desmoid patients was 86 mL (range 6-294 mL), for non-desmoids it was 1 mL. The median percent reduction in targeted lesion volume was 77%, which was significantly beyond our efficacy threshold (p < 0.0001).The median number of sonications for treatment was 87 (range 10-141);median energy per sonication was 1273 J (range 444-2851 J). 3 of the 21 treatments (14%) resulted in first-degree burns; no other adverse events were reported.
Painful lesions were only present in 4 patients. Reduction in pain of greater than 2 points was observed in all patients during 1 to 6 month follow-up.
Discussion
MRgFUS has previously shown promise as an ablative therapy for desmoid tumors in adults [3]. Ablation of vascular malformations in adults has also been published [4]. Previously, Sharma et al [5] published a pilot study of MRgFUS in osteoid osteoma with resolution of pain in 9 patients. However, the ablative efficacy of MRgFUS in a pediatric population has not been evaluated. Our results demonstrate that MRgFUS provides significant reduction in viable lesion volume in all evaluated indications.. Although the sample size of patients with painful lesions was particularly small, data does indicate a reduction in pain based on NRS for the 4 treated painful lesions. Additionally, the large variation of lesion volumes (0.76 – 294 mL) shows the versatility of MRgFUS ablation.Despite the use of skin-cooling mechanisms, minor (first-degree) skin burns did occur. No serious (second or third-degree) burns occurred, no additional adverse effects were reported.
Major limitations of this study include the small patient size, and the indications for treatment were dominated by desmoid fibromatosis. Although durability of treatment would be expected to be in line with other forms of thermal ablation, follow-up is needed to assess the true durability of FUS therapy in children.
Conclusion
MRgFUS provided efficacious ablation in 21 treatment sessions of pediatric patients, with occasional minor skin burns but no serious adverse events. This preliminary data suggests that further investigation of MRgFUS as an ablative option for pediatric patients is warranted.Acknowledgements
No acknowledgement found.References
1. Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010 Feb 1;50(2):221-9.
2. Sharma KV, Yarmolenko PS, Eranki A, Partanen A, Celik H, Kim A, Oetgen M, Kim PC. Magnetic resonance imaging–guided high-intensity focused ultrasound applications in pediatrics: early experience at children's national medical center. Topics in Magnetic Resonance Imaging. 2018 Feb 1;27(1):45-51.
3. Ghanouni P, Dobrotwir A, Bazzocchi A, Bucknor M, Bitton R, Rosenberg J, Telischak K, Busacca M, Ferrari S, Albisinni U, Walters S. Magnetic resonance-guided focused ultrasound treatment of extra-abdominal desmoid tumors: a retrospective multicenter study. European radiology. 2017 Feb 1;27(2):732-40.
4. van Breugel JM, Nijenhuis RJ, Ries MG, Toorop RJ, Vonken EJ, Wijlemans JW, van den Bosch MA. Non-invasive magnetic resonance-guided high intensity focused ultrasound ablation of a vascular malformation in the lower extremity: a case report. Journal of therapeutic ultrasound. 2015 Dec;3(1):1-7.
5. Sharma KV, Yarmolenko PS, Celik H, Eranki A, Partanen A, Smitthimedhin A, Kim A, Oetgen M, Santos D, Patel J, Kim P. Comparison of noninvasive high-intensity focused ultrasound with radiofrequency ablation of osteoid osteoma. The Journal of pediatrics. 2017 Nov 1;190:222-8.
Figures