0494
Assessment of Lung Ventilation and Perfusion of Premature Infants at 1.5 Tesla Using Phase-Resolved Functional Lung (PREFUL) MRI in the NICU1Radiology, Weill Cornell Medicine, New York, NY, United States, 2Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 3Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany, 4Pediatrics, Weill Cornell Medicine, New York, NY, United States, 5Siemens Healthineers, Erlangen, Germany
Synopsis
The purpose of this study was to evaluate quantitative measures of lung function in premature newborns using (PREFUL) MRI on a 1.5 Tesla scanner located within the neonatal intensive care unit (NICU). MRI can non-invasively assess ventilation and perfusion defects in the infant lung without sedation, ionizing radiation or contrast administration. We performed (PREFUL) MRI in 6 neonates [5 preterm/1 term] without respiratory distress to assess lung function in this normal population. Continuation of this work may allow clinicians to quantitatively assess response to treatment in this vulnerable population with respiratory distress.
Introduction
Premature infants represent approximately 10% of all births in developed countries. Bronchopulmonary dysplasia (BPD, also termed chronic lung disease (CLD) of prematurity) is a major respiratory complication of premature birth affecting 10,000 to 15,000 infants in the US annually and up to 50% of infants born < 1,000g.[1] Current techniques assessing structural diagnosis of BPD rely on X-Rays. Rarely CT may also be utilized which involves radiation. Recently, MRI has shown high-quality images of both lung structure and function without any ionizing radiation to the infant.[2]Specifically, we employed a phase-resolved functional lung (PREFUL)post-processing technique on cine spoiled gradient echo (turbo-FLASH) MRI to quantitatively assess lung function.[3] Currently, there are no quantitative means of non-invasively measuring respiratory function in the NICU for infants other than assessing the degree of respiratory support needed for each infant as a surrogate.
Materials & Methods
Five preterm and one term neonate at 38-40 weeks postmenstrual age (3M/3F) were enrolled in a research study incorporating lung MRI (~15 min) when scheduled for a clinical brain MRI. Clinically only one subject had mild bronchopulmonary dysplasia (BPD), others with no evidence of lung disease. No sedation was done. All imaging was performed on a 1.5 Tesla MRI scanner (MAGNETOM Amira; Siemens Healthcare, Erlangen Germany) located within the NICU using neonatal spine and flex coils of an MR compatible incubator (LMT Med Sys;Luebeck,Germany).A single slice 2D spoiled gradient echo (TFL) sequence was acquired with a 168 ms TR, 1.08 ms TE, 5⁰ FA, 10 mm slice thickness, 25 cm FOV and a 128x102 matrix reconstructed to 256x256 resulting in an in-plane resolution of 1 x 1 mm2. 512 images were acquired continuously over 105 seconds with a temporal resolution of 200 ms/frame. No respiratory or cardiac gating was done.
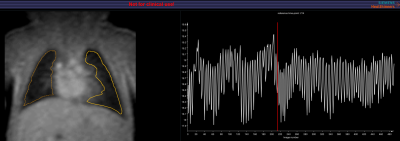
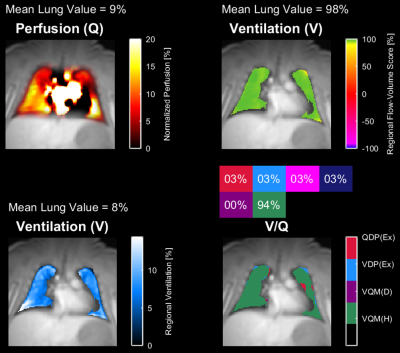
Image analysis was performed in MATLAB using the PREFUL FD analysis routine developed by several of the authors (AV, JVC).[3] Artificial intelligence (AI) segmentation of both lungs was initially run with the ability to manually edit the contour to include additional regions in the periphery.[Figure 1] Motion correction was completed followed by ventilation and perfusion components being filtered and sorted resulting in perfusion maps of the cardiac cycle and ventilation maps of the respiratory cycle.[Figure 2]
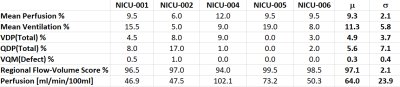
The following parameters were calculated: mean perfusion (Q), mean ventilation (V), perfusion defect % (QDP), ventilation defect % (VDP), V/Q defect and non-defect match %, regional flow-volume score and pulmonary perfusion [ml/min/100mL].[4]
Results
5 of 6 subjects displayed reproducible results as shown in Table 1 (µ±σ): mean perfusion % [9.3±2.1], mean ventilation % [11.3±5.8], total %VDP [4.9±3.7], total %QDP [5.6±7.1], %V/Q defect [0.3±0.4], regional flow-volume score % [97.1±2.1] and pulmonary perfusion [64.0±23.9 ml/min/100mL]. One subject was excluded due to rotational motion that moved the lung in/out of the imaging plane during the scan.Discussion
This technique has previously been applied primarily to adults. However, one group, with several of our co-authors, studied lung function of term neonates at 3.0 Tesla outside the NICU using sedation in a majority of cases.[5] Our study expanded upon this work to image premature infants in the NICU without the need for sedation. The MRI exams were scheduled directly after feeding. The infants were then swaddled and taken directly to the MRI scanner still within the NICU. Our image results show that even at 1.5 Tesla, that the image acquisition can be decreased from the size of an adult lung to that of a premature infant and still maintain sufficient signal to noise and temporal resolution for PREFUL analysis, which can provide potentially sensitive quantified ventilation and perfusion parameters of the neonatal lung.Our results for both ventilation and perfusion are within expected normal ranges. Comparison of %VDP and %QDP with the previous study showed slightly higher values in preterm infants.[5] Pulmonary perfusion values were consistent with those previously published.[4] In addition, the PREFUL technique has previously been validated with both DCE-MRI and 99mTc-HSA SPECT.[6]
One of our subjects was excluded due to rotational motion of the infant during the PREFUL MRI scan. This motion became evident in post-processing as the fraction of lung in the right and left sides became asymmetric. This necessitated viewing of the PREFUL images in real-time as they were being acquired in order to monitor this issue. This procedure prevented any later scans from becoming unusable due to such motion artifacts. Likewise, as the scans are only ~1 minute in length, they may be repeated later in the imaging sequence once the infant has returned to more regular free breathing.
Conclusion
PREFUL lung MRI quantitation is feasible in preterm neonates, without sedation at 1.5 Tesla and may be used in the NICU to evaluate both perfusion and ventilation. Further studies are needed to assess serial changes in BPD and response to treatment.Acknowledgements
No acknowledgement found.References
1) Jensen, E. A., & Schmidt, B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Research Part A: Clinical and Molecular Teratology. 2014;100:145-157.
2) Bamat NA, Zhang H, McKenna KJ, Morris H, Stoller JZ, Gibbs K. The Clinical Evaluation of Severe Bronchopulmonary Dysplasia. NeoReviews, 2020:21;e442-e453.
3) Voskrebenzev A, Gutberlet M, Klimes F, Kairet TF, Schonfeld C, Rotarmel A, Wacker F, Vogel-Claussen J. Feasibility of Quantitative Regional Ventilation and Perfusion Mapping With Phase-Resolved Functional Lung (PREFUL) MRI in Healthy Volunteers and COPD, CTEPH, and CF Patients. MRM 2018;79:2306-2314.
4) Glandorf, J , Klimeš F, Behrendt L, Voskrebenzev A, Kaireit TF, Gutberlet M, Wacker F, Vogel-Claussen J. Perfusion quantification using voxel-wise proton density and median signal decay in PREFUL MRI. Magn Reson Med. 2021;86:1482-1493.
5) Zanette B, Munidasa S, Couch MJ, Stirrat E, Schrauben E, Grimm R, Voskrebenzev A, Vogel-Claussen J, Seethamraju R, Macgowan CK, Greer ML, Tam E, Santyr G. ISMSM 2020, Abstract #0428.
6) Behrendt L, Voskrebenzev A, Klimes F, Gutberlet M, Winther HB, Kaireit TF, Alsady TM, Pohler GH, Derlin T, Wacker F, Vogel-Claussen J. Validation of Automated Perfusion-Weighted Phase-Resolved Functional Lung (PREFUL)-MRI in Patients With Pulmonary Diseases. JMRI 2020;52:103–114.
Figures