0493
Inter- & Intra-visit Reproducibility of Free-Breathing Magnetic Resonance Imaging in Pediatric Cystic Fibrosis Lung Disease1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Siemens Healthcare Limited, Montreal, QC, Canada, 4MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 5MR Collaborations North East, Siemens Healthineers, North East, NY, United States, 6Division of Respiratory Medicine, The Hospital for Sick Children, Toronto, ON, Canada
Synopsis
Free-breathing lung MRI has been shown to be a responsive measure to CF pulmonary exacerbations treatments but have not been used to track stable disease progression longitudinally. In this study we determined the intra- and inter-scan reproducibility of free-breathing lung MRI in healthy and stable pediatric CF across 2 visits and compared to hyperpolarized Xenon MRI (Xe-MRI). Xe-MRI and free-breathing lung show a high intra-scan reproducibility in stable CF subjects, but free-breathing lung MRI showed a low inter-scan reproducibility. However, free-breathing lung MRI significantly correlated with Xe-MRI suggesting that it may be an alternative to expensive, and less wide-spread Xe-MRI.
Introduction
Hyperpolarized 129Xenon-MRI (Xe-MRI) has been shown to capture subtle ventilation defects in lung disease but requires special equipment (i.e. polarizer, coil) and needs the patient to perform a static breath-hold of 7-9 seconds1,2. Free-breathing proton MRI techniques do not require special equipment or breath-holds and therefore may be attractive for the evaluation of pulmonary function, specifically for young children who may not be able to perform coached breathing maneuvers. Free-breathing lung MRI measures local changes in signal arising from lung tissue deformations corresponding to specific phases of respiration (i.e. expiration vs. inspiration)3 captured in 2D slices acquired repetitively during free breathing3,4. In stable Cystic Fibrosis (CF) lung disease, free-breathing lung MRI has been shown to correlate with Xe-MRI5 and capture the treatment effect of antibiotics on a pulmonary exacerbation in CF6, but has not been used to track stable disease progression over multiple visits. As an extension of an ongoing, multi-site study assessing the variability of Xe-MRI and pulmonary function changes after initiation of CFTR modulator therapy (HyPOINT), we assessed the inter- and intra-visit reproducibility of free-breathing lung MRI in children with stable CF lung disease.Methods
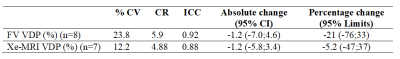
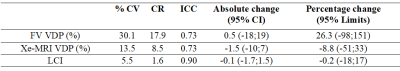
Sixteen stable CF and five healthy controls aged 15±2 years old were imaged in accordance with REB approval at The Hospital for Sick Children. Pulmonary function tests and MRI were performed on all participants on 2 separate visits. Visit 2 occurred one month after. Subjects performed N2 multiple breath washout to obtain lung clearance index (LCI). Multi-slice Xe-MRI and single-slice free-breathing MRI were performed as previously described5,6 and, when time permitted, free-breathing MRI and Xe-MRI acquisitions were repeated in seven and eight CF patients, respectively, within their same visit. Using MATLAB (MathWorks, Natick, MA) fraction ventilation (FV) maps were generated from free-breathing lung MRI based on the phase-resolved functional lung (PREFUL) algorithm described by Voskrebenvez et al.3 with the modification of spatial and temporal filtering steps6. Segmented xenon ventilation distributions were determined from Xe-MRI6. Ventilation defect percentage (VDP) was determined from FV maps using K-means clustering7 and from xenon ventilation distributions using a threshold of 60% of the mean8. The absolute difference, coefficient of variance (% CV), coefficient of reproducibility (CR), intra-class coefficient (ICC), and percentage change were used to calculate inter- and intra-visit reproducibility9. FV VDP, Xe-MRI VDP, and LCI were correlated using linear regression.Results
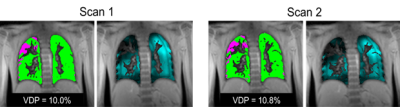
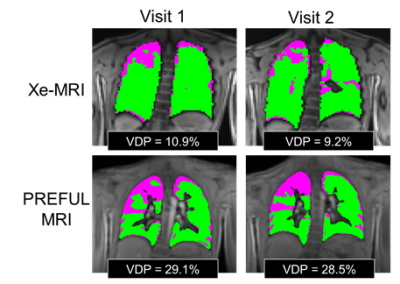
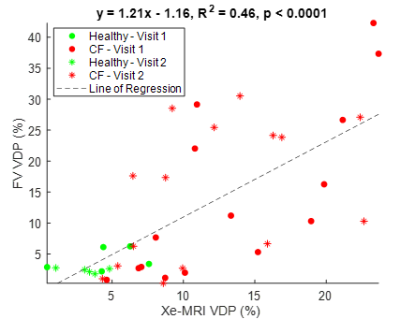
Figure 1 shows the FV maps for a representative CF patient for two different scans taken in the same visit and Figure 2 shows both the Xe-MRI and FV maps for another CF patient taken during 2 separate visits. Table 1 and Table 2 summarize the intra-visit and inter-visit reproducibility of FV VDP, Xe-MRI VDP, and LCI, respectively. FV VDP had a higher %CV, CR, and percentage change as compared to both Xe-MRI VDP and LCI. The within-subject standard deviation of the VDP found from FV VDP across the two visits was found to be proportional to the magnitude of the VDP, while Xe-MRI was not. Of the 16 CF subjects, the mean FV VDP was 13.7±11.7%, mean Xe-MRI VDP was 12.3±5.7% and the mean LCI was 8.1±1.8. Of the 5 healthy subjects, the mean FV VDP was 3.0±1.6%, mean Xe-MRI VDP was 3.4±2.3% and the mean LCI was 5.9±0.3. As shown in Figure 3 FV VDP moderately correlated to Xe-MRI VDP (R2=0.46; p<0.0001). FV VDP also weakly correlated to LCI (R2=0.37; p=0.0001).Discussion
Overall, both the pediatric CF patients' FV VDP and Xe-MRI VDP showed appreciable inter-scan variability between the 2 visits as indicated by the CR values. Similar to LCI9, the within-subject standard deviation in FV VDP between visits was proportional to the mean FV VDP, whereas Xe-MRI VDP was not. The inter-scan reproducibility of Xe-MRI and FV VDP of the 5 healthy participants (CR = 3.5 and 5.3, respectively) was higher than the CF group (CR = 8.5 and 17.9 respectively) during the first two visits, and comparable to the observed intra-scan reproducibility, suggesting the higher variability in the CF group may be due to disease instability/progression. Although there have been limited studies on the reproducibility of MRI within shorter time periods, previous longitudinal studies of Xe-MRI in CF lung disease have shown significant changes in VDP in follow-up visits occurring 1-2 years later10,11. The higher variability of free-breathing lung MRI compared to Xe-MRI and LCI may be apparent because a 2D slice of the lung was analyzed, the placement of which may have varied between visits. Additionally, the movement of ventilation defects to and from the slice of interest may increase variability across visits. Both FV VDP and Xe-MRI VDP showed high intra-scan reproducibility, regardless of disease. There was a moderate and significant correlation between FV VDP and Xe-MRI VDP. The strong correlation was expected since both methods have been shown to detect areas of decreased ventilation1,3,5,6. The sensitivity and reproducibility of a 3D implementation of free-breathing lung MRI will be explored in the future, which is expected to alleviate some of the single-slice shortcomings described here.Conclusion
Free-breathing lung MRI presents a potentially useful alternate method for longitudinal lung disease in CF over Xe-MRI but faces high inter-scan variability.Acknowledgements
We would like to thank the following sources of funding: The Hospital for Sick Children, Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant (RGPIN 217015-2013), the Cystic Fibrosis Foundation (CFF), Canadian Institutes of Health Research (CIHR) operating and project grants (MOP 123431, PJT 153099). Samal Munidasa would like to thank Restracomp and NSERC for their support.References
1. Santyr G, Kanhere K, Morgado F, et al. Hyperpolarized Gas Magnetic Resonance Imaging of Pediatric Cystic Fibrosis Lung Disease. Acad Radiol. 2019;26(3):344-354.
2. Roos JE, McAdams HP, Kaushik S, Driehuys B, Hyperpolarized Gas MR Imaging: Technique and Applications. Magn Reson Imaging C. 2015;23(2):217-229
3. Voskrebenzev A, Gutberlet M, Klimes F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. 2018;79(4):2306-2314.
4. Kaireit TF, Voskrebenzev A, Gutberlet M, et al. Comparison of quantitative regional perfusion-weighted phase resolved functional lung (PREFUL) MRI with dynamic gadolinium-enhanced regional pulmonary perfusion MRI in COPD patients. J Magn Reson Imaging. 2019;49(4):1122-1132.
5. Couch MJ, Munidasa S, Rayment J, et al. Comparison of Functional Free-Breathing Pulmonary 1H and Hyperpolarized 129Xe Magnetic Resonance Imaging in Pediatric Cystic Fibrosis. Acad Radiol. 2021;28(8): e209-e218.
6. Munidasa S, Couch MJ, Rayment J, et al. Free-breathing MRI for Monitoring Ventilation Changes following Antibiotic Treatment of Pulmonary Exacerbations in Pediatric Cystic Fibrosis. Eur. Respir. J. 2021;57(4):2003104
7. Kirby M, Heydarian M, Svenningsen S, et al. 3He magnetic resonance functional imaging semiautomated segmentation. Acad. Radiol. 2012;19(2):141-52
8. Thomen RP, Walkup LL, Roach, DJ, et al. Hyperpolarized 129Xe for investigation of mild cystic fibrosis lung disease in pediatric patients. J. Cyst. Fibros. 2017;16(2):275-282
9. Engberink EO, Ratjen F, Davis SD, et al. Inter-test reproducibility of the lung clearance index measured by multiple breath washout. Eur Respir. J. 2017;50:1700433.
10. Smith L, Marshall H, Aldag I, et al. Longitudinal assessment of children with mild cystic fibrosis using hyperpolarized gas lung magnetic resonance imaging and lung clearance index. Am J Respir Crit Care Med. 2018;197: 397–400.
11. Smith LJ, Horsley A, Bray J, et al. The assessment of short- and long-term changes in lung function in cystic fibrosis using 129Xe MRI. Eur. Respir. J. 2020; doi: 10.1183/13993003.00441-2020.
Figures