0490
Plug-and-play Advanced Magnetic Resonance Spectroscopy
Dinesh K Deelchand1, Pierre-Gilles Henry1, James M Joers1, Edward Auerbach 1, Young Woo Park1, Firat Kara2, Eva Ratai3, Kejal Kantarci2, and Gülin Öz1
1University of Minnesota, Minneapolis, MN, United States, 2Mayo Clinic, Rochester, MN, United States, 3Massachusetts General Hospital, Charlestown, MA, United States
1University of Minnesota, Minneapolis, MN, United States, 2Mayo Clinic, Rochester, MN, United States, 3Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
We developed an automated advanced-single-voxel MRS acquisition protocol at 3T to facilitate acquisition of high-quality spectroscopic data without local MRS expertise. Voxel-based B0 and B1 calibrations were incorporated into the consensus-recommended semi-LASER sequence and combined with automated voxel prescription. Automated B0 and B1 calibrations saved ~4.5min per voxel vs. manual calibrations. All spectra acquired with the automated protocol by rotating MR technologists were usable, while only 83% of those collected with the manual protocol were usable and spectral quality was more variable. The protocol allows automated acquisition of high-quality MRS data with high success rate on a clinical 3T platform.
Introduction
Proton magnetic resonance spectroscopy (1H MRS) allows non-invasive measurements of brain biochemistry and up to 18 metabolites can be quantified in vivo1. MRS complements structural MRI, which provides spatial distribution of water in various tissues. Despite providing noninvasive access to neurochemical changes in a multitude of central nervous system disorders2, MRS has not found wide utility in the clinical setting due to challenges associated with acquiring high-quality and reproducible spectroscopic data. Reliable metabolite quantification from MRS data requires accurate localization of the volume-of-interest (VOI), high signal-to-noise ratio, optimal voxel-based B0 inhomogeneity correction, efficient water suppression (WS) and minimal lipids/ artifacts in the spectrum3. Advanced MRS protocols improve data quality and reproducibility relative to vendor-provided protocols4,5, however, are challenging to incorporate into the clinical workflow and require local MRS expertise for successful implementation6. Here, we developed an automated advanced single-voxel MRS acquisition protocol at 3T to facilitate acquisition of high-quality spectroscopic data without local MRS expertise.Methods
The semi-LASER (sLASER) sequence was recently recommended by MRS experts’ consensus at high fields to minimize CSD artifacts that are prominent with conventional protocol. The compact sLASER sequence7 with interleaved adiabatic refocusing, optimized gradient scheme and 3D outer-volume-suppression (OVS) that was recently harmonized across vendors8 was selected for the automated protocol. First, a B0 shimming protocol was selected for automation by comparing three widely used B0 algorithms; two Siemens-provided protocols: Advanced shim and “Brain” mode shim and FAST(EST)MAP (FM)9. Five healthy participants were scanned at a 3T Prisma scanner and water signal was measured from different six volume-of-interest (VOI) across the brain. The B1 transmit field for all radio-frequency pulses used for WS, OVS and localization in the MRS sequence needs to be correctly set for efficient WS and accurate localization. Voxel-based B1 adjustment consists of arraying the flip angle (FA) (or transmit voltage) of the excitation pulse in the localization sequence and measuring the water signal at each step. These voxel-based B0 and B1 calibrations were incorporated into the sLASER sequence and combined with AutoVOI, a recently developed method for automated voxel prescription. The complete automated advanced MRS protocol is shown in Figure 1. The efficiency of collecting data from a clinical cohort (N=40) with the automated protocol (calibration time and fraction of usable datasets) was compared with the non-automated semi-LASER protocol (N=46) whereby all prescan calibrations were executed manually in the academic hospital setting with rotating MR technologists in the neuroradiology unit6. MRS data were collected from the bilateral posterior cingulate cortex (8 mL) using sLASER and FM shimming on a Siemens 3T.Results
A multi-iteration FAST(EST)MAP protocol resulted in narrower water linewidths than vendor’s B0 shim protocols for data acquired from six brain locations (P<1e-5) and was selected for automation (Figure 2). Since the water linewidths were narrowest and within 10Hz for all VOIs with FM (P<1e-5 vs. vendor routines), FM was therefore incorporated into the automated MRS protocol. The time taken for manual B0 and B1 calibrations ranged from 5 to 15.5min with a mean time of ~8min. In contrast, the time taken was identical (~4min) for all subjects in the automated MRS protocol, demonstrating an average gain of ~4.5min per VOI (P<1e-5) compared to the manual protocol. All spectra acquired with the automated protocol were usable, while only 83% of those collected with the manual protocol were usable (N=38). After exclusion of unusable spectra, comparable spectral quality was observed between protocols (Figure 3), with higher variation in water residual signal with the manual protocol. Although similar water linewidths (mean±standard deviation, 6.1±2.0Hz with manual vs. 6.7±0.5Hz with automated) were observed in PCC with both protocols, the between-subject coefficients-of-variation was larger with the manual protocol (33% with manual vs. 8% with automated protocol) due to higher variation of linewidth in manual protocol.Discussion
This study showed that an automated advanced MRS protocol can be implemented on clinical scanners whereby both B0 and B1 adjustments are executed without operator intervention. The protocol yields savings in data acquisition time, increases the rate of successfully acquired datasets and improves between-subject consistency in spectral quality compared to the same protocol executed manually where the MR operator needs to set the 1st and/or 2nd order shim values after each FM iteration and also determine and input the optimal transmit voltage and WS-FA. This is a very important step to facilitate streamlined MRS acquisition in research and clinical settings. A limitation of the current study was that the automated protocol was only evaluated for single-voxel MRS. However, the same automated principles can be applied to multi-voxel MRS imaging10 where B0 and B1 adjustments would be carried out on a larger voxel or a whole slice depending on the sequence utilized. An advantage of the proposed automated protocol is that its implemented is not restricted to sLASER, but it has potential to be implemented for other advanced MRS sequences at various fields. Although, this automated approach was demonstrated on a Siemens scanner here, this technique could be implemented on other vendors’ platform.Conclusion
The plug-and-play advanced MRS protocol allows automated acquisition of high-quality MRS data with high success rate and consistency on a clinical 3T platform.Acknowledgements
This work was supported by funding from the National Institutes of Health (NIH) (R01 NS080816, P41 EB027061, P30 NS076408). We would like to thank the MR technologists at the Mayo Clinic for the MRS data acquisitions and all study participants.References
- Pfeuffer J et al. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time 1H NMR spectra of the rat brain. J Magn Reson 1999;141(1):104-120.
- Öz G et al. The MRS Consensus Group. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014;270(3):658-679.
- Tkáč I & Gruetter R. Methodology of 1H NMR spectroscopy of the human brain at very high magnetic fields. Appl Magn Reson 2005;29(1):139-157.
- Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednařík P, Eberly LE, Öz G. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med 2016;76(4):1083-1091.
- Deelchand DK et al. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med 2015;73(5):1718-1725.
- Deelchand DK et al. Improved localization, spectral quality, and repeatability with advanced MRS methodology in the clinical setting. Magn Reson Med 2018;79(3):1241-1250.
- Öz G & Tkac I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: Validation in the cerebellum and brainstem. Magn Reson Med 2011;65(4):901-910.
- Deelchand DK et al. Across-vendor standardization of semi-LASER for single-voxel MRS at 3T. NMR in Biomedicine 2021;34(5):e4218.
- Gruetter R & Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med 2000;43(2):319-323.
- Maudsley AA et al. Advanced magnetic resonance spectroscopic neuroimaging: Experts' consensus recommendations. NMR in Biomedicine 2021;34(5):e4309.
Figures
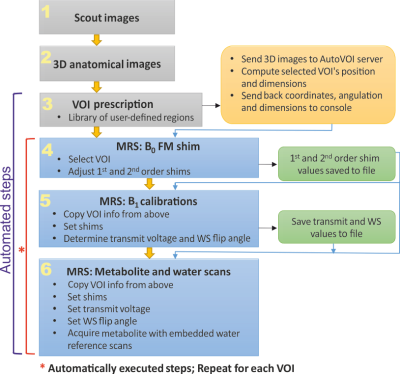
Flowchart shows the different steps in the automated advanced MRS
pipeline. Gray boxes represent images acquired and used to define the MRS VOIs
to use in the study. Blue boxes represent the MRS protocol that includes B0
shim correction and flip angle B1 calibrations followed by
metabolite and water reference acquisitions. Yellow box represents the AutoVOI
pipeline used to prescribe the MRS VOIs. Green boxes represent shim and
calibration information saved to file on the console and used by subsequent steps
in the MRS protocol, as depicted by the blue arrows.
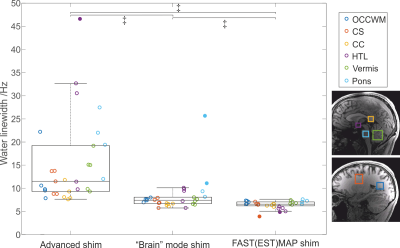
Water linewidth (in Hz) measured using three different B0
shim techniques (Advanced, “Brain” and FM shims) in 5 subjects at 3T. Linewidth
was measured using the sLASER sequence from the occipital white matter (OCCWM,
18x18x18 mm3), splenium of the corpus callosum (CC, 25x13x13 mm3),
centrum semiovale (CS, 15x20x25 mm3), hypothalamus (HTL, 12x13x10 mm3),
vermis (10x25x25 mm3), pons (15x15x15 mm3). ‡ P<1e-5 (Wilcoxon test).
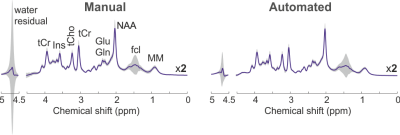
sLASER proton spectra (mean±standard deviation, TR/TE=3000/30 ms)
measured from the posterior cingulate cortex at 3T with a manual (N=38) and an
automated (N=40) advanced MRS protocol. Due to different
levels of falx cerebri lipid (fcl) signal in older adults, a variation in
signal at 1.45 ppm was observed in both cases.
DOI: https://doi.org/10.58530/2022/0490