0487
MRI-based cortical shear strain measurement in healthy volunteers: repeatability study and its implications for sub-concussive trauma1Radiology, Mayo Clinic, Rochester, MN, United States, 2Physiology and Biomedical Engineering, Mayo Clinic, Rochester, MN, United States
Synopsis
Repetitive head impact (RHI) increases the risk of concussion, probably due to alterations of the brain-skull mechanical coupling. It would be of clinical value to have a reliable biomarker capable of assessing the status of the brain-skull coupling condition. MRE-based normalized octahedral shear strain (NOSS) has been proposed as a method for such brain membrane system evaluation. To verify the test-retest reliability of NOSS, datasets from 14 healthy volunteers were acquired with different vibration directions and within- and between-day scanning. This study demonstrated the excellent repeatability of the proposed method, showing the NOSS distributions were robust to loading variations.
Introduction
Sub-concussive, repetitive head impact (RHI) is emerging as a public health issue, and there is a need for a robust method to evaluate the effect of RHI exposures.1 Our previous study used an MR elastography (MRE) based method to visualize and quantify normalized octahedral shear strain (NOSS) on the cortical surface.2 We found consistency between the density of arachnoid trabeculae reported in the literature, that has a considerable effect on brain mechanics during head impact,3 and our NOSS distributions. Cortical NOSS may thus be a potential biomarker for measuring the skull-brain mechanical coupling and its potential alterations in response to RHI. However, the repeatability of this method has not been investigated. The purpose of this study was to evaluate the reliability of this MRE-based cortical shear strain assessment method under different scanning conditions and provide a robust brain-skull coupling parameter for assessing the ability of the membrane system to protect the brain from external impacts.Method
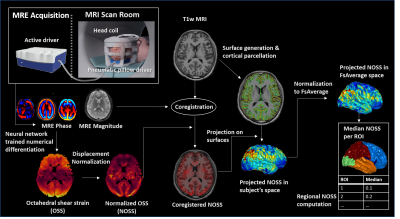
After IRB approval and written informed consent, 14 healthy volunteers (age 40.21±13.01yrs, 6F/8M) were recruited for this study. The brain MRE exams were performed on a compact 3T MR scanner with a dual-sensitivity and dual-motion encoding (DSDM) MRE pulse sequence.4 60Hz vertical or horizontal vibrations were introduced into the brain via a pneumatic driver. For the repeatability test, 14 volunteers were imaged under vertical vibrations (AP direction), and 6 of them were additionally scanned under horizontal vibrations (LR direction). All the examinations were repeated 3 times with one baseline scan during the first visit, and two repeated scans separated by a 5-minute break leaving the scanner after one month. Octahedral shear strain (OSS) was computed using a neural network trained numerical differentiation5 and normalized (NOSS) by the wave displacement amplitude. FreeSurfer was used to generate the cortical surface and co-register MRE data to anatomical images. The mean OSS/NOSS across the cortical ribbon was overlaid on the cortical surface in subject space and then projected to FsAverage space. The median OSS/NOSS in each region of interest (ROI) was calculated for statistical analysis. The data processing workflow is illustrated in Fig.1. A paired two-sided Wilcoxon signed-rank test was performed to identify differences between different vibration directions (P-value < 0.05 was considered statistically significant). The pixel-wise and region-based intraclass correlation coefficients (ICCs) were also calculated for evaluating the within-day and between-day repeatability of NOSS under the same AP vibration pattern.Results
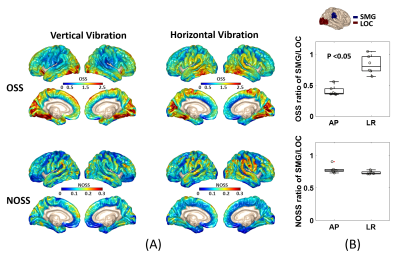
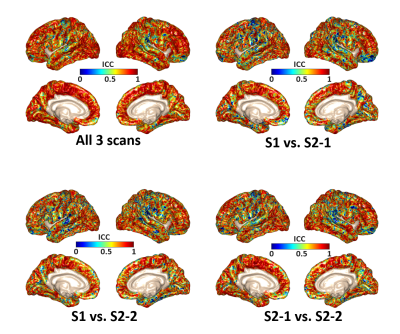
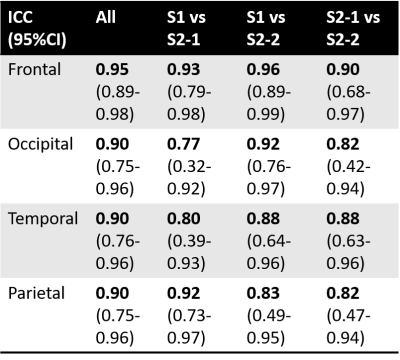
Fig.2 shows the averaged OSS/NOSS maps across six subjects. The OSS maps show elevated values at the driver locations, while these regional variations are greatly reduced by amplitude normalization. The statistical results reveal a significant difference between the two vibration modes in the OSS ratio of the supramarginal gyrus/lateral occipital cortex (SMG/LOC) that are close to the driver locations (p<0.05). However, there was no significant difference in NOSS between the AP and LR vibrations. The pixel-wise ICC values of NOSS across the three different MRE scans with AP vibrations are overlaid on the cortical surface (shown in Fig.3). Both the within-day (S2-1 vs. S2-2) and between-day (S1 vs. S2-1, S1 vs. S2-2) results show good to excellent repeatability in most regions. Table 1 summarizes repeatability results in different cortical lobes. These results also indicate high test-retest reliability with a minimum ICC greater than 0.77.Discussion
This test-retest study demonstrated the high reliability of the MRE-based cortical strain assessment in response to different non-impact dynamic loadings. The vibration amplitude, shear wave propagation direction, and transmission efficiency of the external loading may vary substantially between examinations, which may lead to inconsistent estimates of specific metrics, such as OSS (Fig.2 B). In contrast, our results showed that NOSS was less sensitive to the experimental setup, suggesting NOSS is a more robust biomarker for evaluating the skull-brain coupling changes. However, we still observed that the temporal lobe tends to retain higher NOSS variations when changing the vibration direction, and the pixel-wise ICCs in this area are lower than in other regions. This may be partly due to susceptibility-induced distortion in the EPI pulse sequence and could be improved by applying susceptibility distortion correction before registration. In a future study, we will also collect more data to analyze the dependence of cortical shear strain on age and sex. The patterns of cortical NOSS may suggest a potential mechanism of the local vulnerability of certain cortical regions due to the weakness of the brain membrane system.Conclusion
This study investigated the repeatability of cortical shear strain measurements in healthy volunteers and showed that NOSS can be used as a reliable parameter to evaluate the strain of the cortical surface under non-impact dynamic loading. It provides a new perspective to explore the brain-skull decoupling mechanism of protecting the brain from external impacts and the potential clinical application for assessing the status of the brain membrane system under sub-concussive, repetitive head impact.Acknowledgements
This work was supported by grants from the NIH (R01EB001981, R01 NS113760, and U01EB02445).References
1. Mainwaring L, Ferdinand Pennock KM, Mylabathula S, Alavie BZ. Subconcussive head impacts in sport: A systematic review of the evidence.International journal of psychophysiology: official journal of the International Organization of Psychophysiology 2018.
2. Yin Z, Murphy M, Sui Y, Manduca A, Ehman RL, Huston III J. MRI-based assessment of regional patterns of cortical strain in the human brain resulting from non-impact dynamic mechanical loading. 2021; ISMRM Virtual Conference.
3. Benko N, Luke E, Alsanea Y, Coats B. Spatial distribution of human arachnoid trabeculae. Journal of Anatomy 2020;237(2):275-284.
4. Yin Z, Sui Y, Trzasko Joshua D, Rossman Phillip J, Manduca A, Ehman Richard L, Huston J. In vivo characterization of 3D skull and brain motion during dynamic head vibration using magnetic resonance elastography. Magn Reson Med 2018;0(0).
5. Murphy M, Trzasko J, Scott J, Manduca A, Huston III John, Ehman R. Artificial neural networks for numerical differentiation with application to magnetic resonance elastography. 2020; ISMRM Virtual Conference.
Figures