0486
Develop CEST-detectable liposomes for nose-to-brain drug delivery1Department of Biomedical Engineering, City University of Hong Kong, Kowloon, Hong Kong, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3City University of Hong Kong Shenzhen Research Institute, Shenzhen, China, 4Hong Kong Centre for Cerebro-Cardiovascular Health Engineering (COCHE), Hong Kong, Hong Kong
Synopsis
Nose-to-brain-drug-delivery is an effective route to administer drug to the brain bypassing the blood-brain-barrier(BBB). No non-invasive way to assess the delivery-efficiency available yet, especially with brain distribution. Here we investigated the imaging of nanomedicine-delivery via intranasal-administration using CEST-detectable(Iohexol loaded)mucus-penetrating-liposome(with10%PEG). The mucus-retention and penetration-efficiency was examined by in-vitro cell study and in-vivo CEST MRI after injecting the Iohexol-loaded-mucus-penetrating-liposome(Ioh-Lipo). 10%PEG-Ioh-Lipo showed a 20%higher mucus-retention than 1%PEG-Ioh-Lipo. This was also observed in-vivo with significant CEST-contrast difference in the OB between 3groups of mice at 4.3 and 3.5ppm. Moreover, liposomes were mainly found in the ONL, EPL, and MCL of the OB in histology.
Introduction
Intranasal drug delivery provides an alternative route to enhance the efficiency of drug delivery to the brain via bypassing the BBB1. Many studies have reported increases in the effective dose to the brain by using intranasal administration when compared to oral or intravenous injection2,3,4. As previously reported, the intranasal drug delivery to the brain via several pathways, such as intracellular, extracellular, glymphatic pathway and other likes through systemic circulation5,6,7. Currently, there is lack of non-invasive methods for monitoring the drug delivery pathway and drug distribution in the brain after administration in-vivo. Chemical exchange saturation transfer(CEST) could be used to monitor liposome-based nanomedicine, biodistributions and potentially the drug delivery efficiency and pathway8,9. Mucus with a mesh size around 150-200nm is the only barrier in the intranasal cavity to block the delivery of liposome from nose-to-brain1. Previous study has showed that ≥7mol%-PEG liposomes have mucus penetrating property compared to 0and3mol%-PEG liposomes10. Many studies have showed CT contrast agent can be a CEST contrast agent because of the existent of the amide protons11,12,13. Herein, we developed a mucus-penetrating-liposome loaded with CT contrast agent to image the intranasal drug delivery. The CEST contrast of Iohexol-loaded-liposome(Ioh-Lipo) at 4.3ppm could be monitored in olfactory bulb and frontal lobe after intranasal administration at 3T. The liposomes mainly distributed to distinctive regions in the olfactory bulb. This approach is robust in monitoring of drug distribution in the brain, its drug delivery efficiency and potential delivery pathway into the brain.Methods
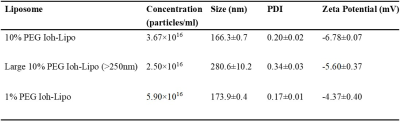
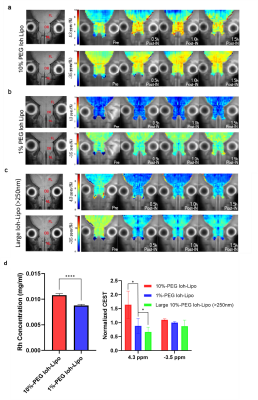
Ioh-lipo was prepared through thin-film-hydration method14. Human nasal epithelial cell RPMI2650 is used for liposome retention cell study. The cells were maintained in DMEM supplemented with 10%FBS and 1%Penicillin-Streptomycin at 37°C in a >95% humidified atmosphere of 5%CO2 in air for 14 days after 100% confluent for mucus secretion. 10%Ioh-Lipo and 1%Ioh-Lipo is diluted with the cell culture media and added in each well for 30mins then washed by PBS before measuring the fluorescence reading by microplate reader. 150ul of Ioh-lipo was administrate intranasally by pipette into right nostril of ICR mouse15. The in-vivo MRI images for intranasal administration experiments at the olfactory bulb of mice were acquired pre-injection, 0.5hr,1hr,1.5hr after Intranasal injection on a horizontal bore 3T Bruker BioSpec system equipped with a 40-mm volume transceiver coil using a modified rapid acquisition with relaxation enhancement(RARE) sequence(Slice thickness=1.5 mm, field of view(FOV)=16x16 mm, image size=64x64, RARE factor=18, repetition time/echo time(TR/TE)=5000/5 ms, B1=0.6uT, -20to+20 ppm, 0.2ppm steps with an extra acquisition point on ±3.5,4.1,4.3ppm). T2 images showed the olfactory bulb and frontal lobe. CEST Z-spectra were acquired and CEST contrast was calculated by applying Lorentzian fitting on Z spectra (CEST contrast%) for analysis of penetrating efficacy by intranasal administration and the potential pathway in the nasal turbinates and the olfactory bulb in treated ICR mouse. CEST contrast at 4.3 and -3.5 ppm 1hr after administration was normalized to pre-injection to compare the differences among the three liposome formulations in Fig.2d. One-way anova statistical test was used for the comparison, and p-value < 0.05 was considered as significant.Results and Discussion
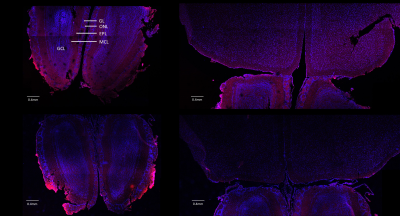
We prepared Ioh-Lipo with two different mol% of PEG, i.e. at 1% and 10% to perform the mucus retention study. We also prepared Ioh-Lipo with different size, i.e.<200nm and>250nm to perform the in-vivo CEST imaging. The liposome characterization can be found in Table1. In vitro study, we observed a 20% higher retention in the mucus of RPMI2650 cell of 10%mol PEG Ioh-Lipo than in 1mol% PEG Ioh-Lipo(Fig.1c). This supports an improved mucus retention of liposomes with high PEG content, which were also reported in previous studies10,14. A 53.7% increase in CEST contrast at 4.3ppm and 10.0% increase in CEST contrast at -3.5ppm were observed an hour after administration in olfactory bulb, Mice received 10%PEG Ioh-Lipo showed a significant increase of CEST contrast at 4.3ppm in olfactory bulb and frontal lobe after intranasal administration when compared with mice received large size 10%PEG Ioh-Lipo and 1%PEG Ioh-Lipo (P<0.01;P<0.001,Fig.1d). Together with the in vitro mucus retention of 10%PEG Ioh-Lipo, this indicates that a high PEG content of liposomes improved mucus retention and penetration property for nose-to-brain delivery. Moreover, a much lower CEST contrast at 4.3 ppm was observed in 10% PEG Ioh-Lipo with size larger than 200nm(Fig.1d), which indicated that only liposomes smaller than 200nm can go through the nasal mucus of mesh size around 150-200nm1. Histology study(Fig.3) showed the distribution of rhodamine-labeled liposomes in the olfactory bulb and frontal lobe. We can also observe that liposomes also highly accumulated in edge between the olfactory bulb and the frontal lobe. Notably, in the olfactory bulb, we observed that liposomes mainly appeared in the ONL, EPL and the MCL. These distributions could indicate liposomes were delivered to the brain via intracellular pathoway and/or glymphatic pathway1. This unique CEST contrast and mucus penetrating property of Ioh-Lipo could facilitate image-guided nose-to-brain drug delivery, the size dependence and the potential pathway of intranasal drug delivery could facilitate further development of nanocarriers for intranasal drug delivery.Conclusion
Here, we developed and monitored the CEST-detectable brain-administrable mucus-penetrating intranasal liposome. We assess the nose-to-brain delivery efficiency and monitor the drug distribution Ioh-Lipo inside the brain and predicted the possible pathway of intranasal drug delivery.Acknowledgements
This work was supported by the Research Grants Council: 11102218; City University of Hong Kong: 7005210, 7005433, 9680247, 9667198 and 9609307; National Natural Science Foundation of China: 81871409.References
1. Crowe TP, Greenlee, M. H., Kanthasamy, A. G., & Hsu, W. H. Mechanism of intranasal drug delivery directly to the brain. Life Sciences,195, 44-52, ((2018)).
2. Chovan JP, Klett RP, Rakieten N. Comparison of meclizine levels in the plasma of rats and dogs after intranasal, intravenous, and oral administration. Journal of pharmaceutical sciences 74, 1111-1113 (1985).
3. Banks WA, Morley JE, Niehoff ML, Mattern C. Delivery of testosterone to the brain by intranasal administration: comparison to intravenous testosterone. Journal of drug targeting 17, 91-97 (2009).
4. Westin UE, Boström E, Gråsjö J, Hammarlund-Udenaes M, Björk E. Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharmaceutical research 23, 565-572 (2006).
5. Sun B-L, et al. Lymphatic drainage system of the brain: a novel target for intervention of neurological diseases. Progress in neurobiology 163, 118-143 (2018).
6. Norwood JN, Zhang Q, Card D, Craine A, Ryan TM, Drew PJ. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. Elife 8, e44278 (2019).
7. Liu H, et al. Olfactory route for cerebrospinal fluid drainage into the cervical lymphatic system in a rabbit experimental model. Neural regeneration research 7, 766 (2012).
8. Van Zijl PC, Yadav NN. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magnetic resonance in medicine 65, 927-948 (2011).
9. Zhang XY, et al. Accuracy in the quantification of chemical exchange saturation transfer (CEST) and relayed nuclear Overhauser enhancement (rNOE) saturation transfer effects. NMR in biomedicine 30, e3716 (2017).
10. Yu T, et al. Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI). Nanomedicine: Nanotechnology, Biology and Medicine 11, 401-405 (2015).
11. Aime S, et al. Iopamidol: Exploring the potential use of a well‐established x‐ray contrast agent for MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 53, 830-834 (2005).
12. Moon BF, et al. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extracellular pH. Contrast media & molecular imaging 10, 446-455 (2015).
13. Anemone A, Consolino L, Longo DL. MRI-CEST assessment of tumour perfusion using X-ray iodinated agents: comparison with a conventional Gd-based agent. European radiology 27, 2170-2179 (2017).
14. Chan KW, et al. A diaCEST MRI approach for monitoring liposomal accumulation in tumors. Journal of controlled release 180, 51-59 (2014).
15. Hanson LR, Fine JM, Svitak AL, Faltesek KA. Intranasal administration of CNS therapeutics to awake mice. Journal of visualized experiments: JoVE, (2013).
Figures