0480
Cardiac cycle and flow compensation effects on REnal Flow and Microstructure AnisotroPy (REFMAP) MRI in healthy human kidney
Eric Sigmund1, Artem Mikheev1, Inge Brinkmann2, Thomas Benkert3, and Hersh Chandarana1
1Radiology, NYU Langone Health, New York, NY, United States, 2Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
1Radiology, NYU Langone Health, New York, NY, United States, 2Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 3Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Diffusion-weighted imaging in renal tissue is a complex interplay of microstructural and microcirculation effects, which are often quantified with diffusion tensor imaging (DTI) or intravoxel incoherent motion (IVIM), as well as hybrid models such as Renal Flow and Microstructure AnisotroPy (REFMAP). This work measures the modulation of these effects with cardiac phase and with flow compensated (FC) diffusion gradient waveforms. Results show that both cardiac phase and FC affect diffusion metrics signficantly in cortex and medulla, and suggest that these experimental tools may in the future be leveraged to increase biophysical specificity of renal diffusion MRI metrics.
Purpose
Diffusion MRI (DWI) contrast in the kidney involves an interplay of microstructural hindrances and microcirculation (1,2). Two representations are diffusion tensor imaging (DTI)(3,4), which measures directional anisotropy of water motion, and intravoxel incoherent motion (IVIM) (5,6), which separately quantifies microcirculation and microstructural restriction. Some hybrid methods jointly measure flow and structural anisotropy (7-9). Tubular and vascular flow both contribute to apparent perfusion fraction, and some studies have suggested that they can be resolved via rate of pseudodiffusion with multicompartment analysis (10-13). Renal DWI has also been shown to be modulated with cardiac cycle (14-18) and with flow-compensated diffusion gradients (19). These tools provide both means of contrast and opportunities for modeling. We show cardiac gated REnal Flow and Microstructure AnisotroPy (REFMAP) data with and without flow compensation in healthy volunteers to explore its full range of contrast.Methods
In this HIPAA-compliant and IRB-approved prospective study, 8 volunteers (7 F, 1M ages 22-54) provided written informed consent and had their abdomens imaged in a 3 T MRI system (MAGNETOM Prisma; Siemens Healthcare, Erlangen, Germany) in supine position with posterior spine array and anterior body array RF coils and chest leads for ECG gating. Coronal oblique T2-weighted HASTE images were collected for anatomical reference. Sagittal phase contrast (PC) MRI images through the left renal artery were collected at multiple cardiac phases to estimate pre-systolic, systolic, and diastolic phases for kidney tissue. With a prototype advanced diffusion weighted imaging sequence with dynamic field correction, cardiac triggered oblique coronal DWI (TR/TE 2800/81 ms, matrix 192/192/1, resolution 2.2/2.2/5 mm) were collected at 10 b-values (b=0, 10,30,50,80,120,200,400,600,800 s/mm2) and 12 directions, with both bipolar and flow-compensated diffusion gradients. The same protocols were acquired from a static water phantom. Acquisitions were performed at maximal velocity (systole, 24±6% full cycle), late stable velocity (diastole, 73±5% full cycle), and between trigger point and systole (pre-systole, 9±2% full cycle). Kidneys were individually cropped and mutual information used for motion correction (Firevoxel v356), and further analyzed with custom code (Igor Pro 8.0). REFMAP analysis generated diffusion tensor metrics (λi, MD, FA) for the slow tissue diffusivity, scalar perfusion fraction fp, and mean, axial, and radial pseudodiffusion (Dp) for both cortex and medulla (segmented on b0 and FA maps respectively). Isotropic averaged integrated signal decays vs. b-values were measured in phantom. Velocity waveforms were extracted from the renal artery in PC-MRI data and velocities were extracted from the peak point and at diastole. Independent samples t-tests and one-way ANOVA (SPSS 25.0) evaluated group dependencies on cardiac cycle and flow compensation. Pearson correlation coefficients were measured between left/right averages of diffusion metrics and renal artery velocities, both in absolute terms and as systolic/diastolic ratios.Results
6 kidneys were omitted from analysis due to poor positioning, throughplane motion, or variable distortion, leaving 10 kidneys in 6 subjects. Figure 1 shows example PC-MRI slice location and velocity waveform in the renal artery and corresponding diffusion metrics and maps in the adjacent left kidney at multiple trigger delays. Figure 2 shows example integrated signal decays for each pulse sequence. Water shows identical monoexponential decay for both sequences, while renal cortex at systole shows higher perfusion fraction for the bipolar case. One-way ANOVA showed all parameters except MD were significantly different between bipolar and flow compensated cases, with the largest effect sizes appearing in FA and fp (Figure 3). Conversely, only λ2, MD, and mean/axial/radial Dp showed significant variance with cardiac phase in the global dataset, and with smaller effect sizes than flow compensation. Average Dp in cortex and medulla correlated significantly with arterial velocity. Sys/dias ratios of fp and FA in cortex and medulla correlated significantly with sys/dias ratios of arterial velocity (Figure 4).Discussion
The present study indicates that the flow contribution to kidney DWI is affected differently by cardiac cycle and flow compensation. As previously observed, diffusion metrics (particularly those associated with pseudodiffusion) correlate with renal artery blood velocity. The majority contribution to perfusion fraction remains constant with cardiac cycle, with an additional pulsatile component; this may be consistent with tubular and vascular flow respectively. Flow compensation dramatically reduces both compartments but constant and pulsatile fractions remain; this indicates flow compensation filters some flow contributions from both vascular and tubular spaces. The slow compartment shows some cortical pulsatility in MD and significantly reduced medullary FA with flow compensation, suggesting dynamics remains relevant in that compartment. Diffusion time differences between the two waveforms may also play a role.Conclusion
Cardiac gating and flow compensation induce significant modulation of diffusion and flow anisotropy in the human kidney. Future signal modeling may shed further biophysical light on these contrast variations.Target audience
Scientists and clinicians interested in diffusion contrast in renal tissueAcknowledgements
No acknowledgement found.References
1.Caroli A, Nephrol Dial Transplant 2018; 2. Sigmund EE, Radiology 2012; 3. Ries M JMRI 2001; 4.Basser PJ. NMR biomed 1995; 5.Le Bihan D, Radiology 1986; 6.Muller MF, Eur J Radiol 1998;7.Notohamiprodjo M, MRM 2015;8.Hilbert F, NMR biomed 2016;9.Liu AL JMRI 2018;10. Baalen Sv, JMRI 2017; 11. Periquito JS, QIMS 2021; 12. van der Bel R, Eur J Radiol 2017;13. Stabinska J, MRM 2021;14. Lanzman RS, MRI 2019;15. Wittsack HJ Invest radiol 2012;16. Heusch P, JMRI 2013;17. Ito K, MRI 2018;18. Milani B MRM 2019 19. Wetscherek A,MRM 2015.Figures
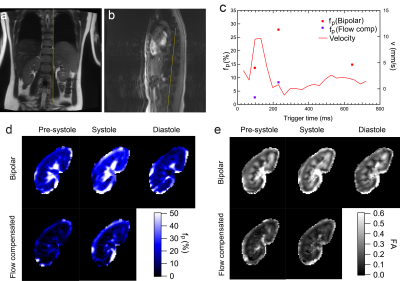
Fig.
1 : (a) T2w-HASTE; (b) PC-MRI magnitude; (c) renal
artery velocity / cortical perfusion fraction fp; (d) fp
maps; (e) Fractional anisotropy (FA) maps from REFMAP analysis at several
cardiac phases and bipolar / flow compensated sequences in a volunteer.
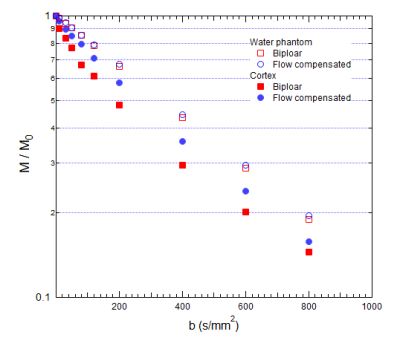
Fig.
2: Isotropic average signal decays in water and renal
cortex for bipolar / flow compensated sequences.
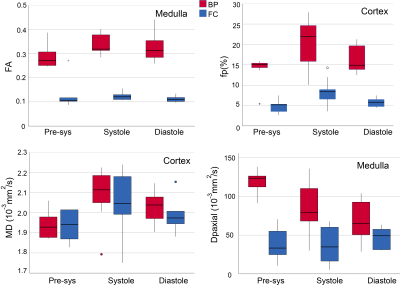
Fig. 3: Group
results of FA, fp, MD, and Dpaxial in phases and sequences
(bipolar (BP) / flow compensated (FC)).
FA and fp show dramatic decreases with flow compensation with
some residual pulsatility. MD shows
minimal pulse sequence dependence but clear pulsatility. Dp,axial
shows phase and sequence sensitivity
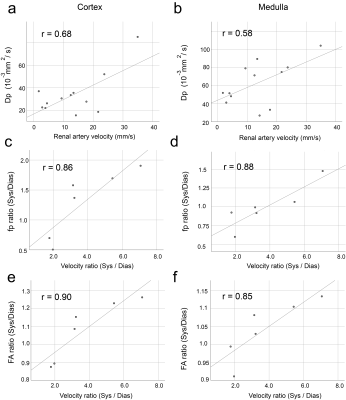
Fig. 4. Significant correlations between diffusion metrics
and renal artery velocity in cortex (a,c,e) and medulla (b,d,f). Absolute pseudodiffusivity Dp correlates with
absolute renal artery velocity (a,b). Systolic
/ diastolic ratios of perfusion fraction
fp (c,d) and fractional anisotropy FA (e,f) correlate significantly with those
of arterial velocity.
DOI: https://doi.org/10.58530/2022/0480