0475
Differentiation of tumor grade for renal cell carcinoma using MR Fingerprinting1Radiology, Case Western Reserve University, Cleveland, OH, United States, 2Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 3Pathology, Case Western Reserve University, Cleveland, OH, United States, 4Pathology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 5Urology, Case Western Reserve University, Cleveland, OH, United States, 6Urology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Synopsis
In this study, we applied kidney MR Fingerprinting in characterization of renal cell carcinoma (RCC) and correlated the quantitative T1 and T2 values with tumor grade. Our results show significantly lower T2 values between the high-grade/unclassified RCC group and the chromophobe/low-grade RCC group (55±19 vs. 90±24 msec; P<0.05), while no significant difference in T1 values was noticed (P>0.05). More importantly, MRF T1 and T2 measurements provide complementary information in characterization of RCC tumor grades with a sensitivity of 89% and a specificity of 98% in differentiation of the two RCC groups, demonstrating the strength of multi-parametric imaging.
Introduction
Renal cell carcinoma (RCC) is the most common malignant renal neoplasm, which often presents as an incidentally detected, incompletely characterized renal mass (1). Due to the limited capability of current imaging techniques, many of these patients with incidental renal mass undergo direct surgery or biopsy without further imaging evaluation which adds to unnecessary morbidity and health care cost (2). Recent studies have shown that MR tissue relaxometry mapping including T1 and T2 relaxation times can provide improved characterization of kidney diseases and correlate with tumor grade and biologic aggressiveness in RCC (3, 4). However, the current kidney relaxometry mapping techniques still suffer from long breath-holds, limited spatial resolution, and ability to mostly capture one tissue property at a time. We have recently developed a kidney MR Fingerprinting technique which can provide simultaneous T1 and T2 quantification in ~15 seconds per slice and reduced sensitivity to B1 inhomogeneities (5). In this study, we aim to evaluate the MRF technique in characterization of RCC and correlate the quantitative T1 and T2 values with tumor grade.Methods
In this IRB-approved HIPAA compliant prospective study, 13 adult (>18years) patients (M:F, 8:5; mean age, 60 (range 41-77) years) with suspected RCC > 1cm in size, without prior biopsy and with surgery within 6 months of MRI were enrolled between November 2020 and August 2021. All MRI exams were performed on a 3 T MRI unit (MAGNETOM Vida, Siemens Healthineers, Erlangen, Germany) and 2D breath-hold kidney MRF was applied on each subject. The imaging parameters for MRF included: FOV, 40×40 cm; matrix size, 256×256; slice thickness, 5 mm; flip angles, 5°~12°; number of time frames, ~1700. For each subject, multiple MRF scans in the coronal plane were acquired and each scan took ~15 seconds.MRF reconstruction was performed offline on a standalone workstation (12 core, 2.6GHz Intel Xeon E5-2630 v2 processor; and 128GB RAM). Co-registered ROI analysis by abdominal radiologist was performed to obtain mean T1 and T2 values in the solid areas of the tumor. Tumor subtype and ISUP grading (grade 1/2 - low-grade and grade 3/4 - high-grade) for RCC were recorded. Statistical analysis was performed to evaluate the capability of the acquired quantitative tissue properties (T1 and T2) in differentiation of RCC tumor grade.
Results
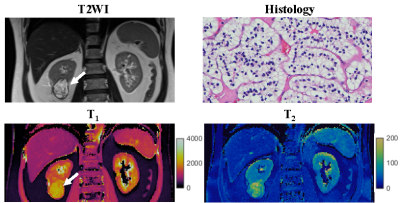
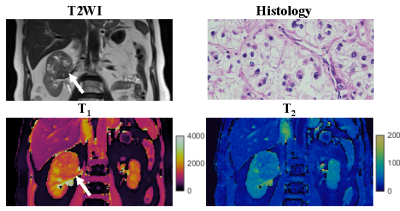
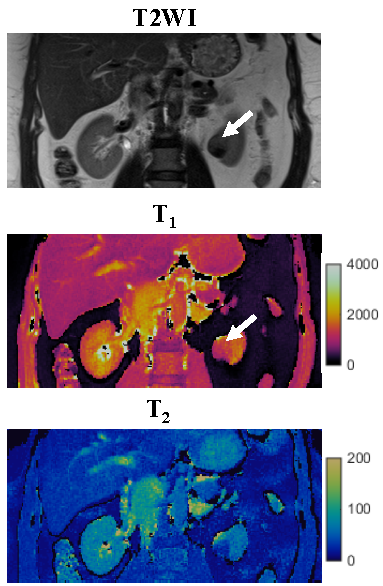
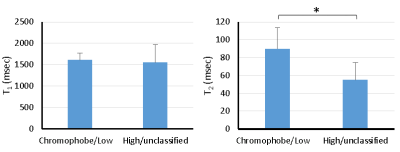
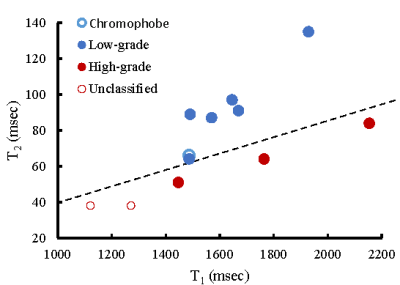
One patient was excluded due to failure of MRF. Of the remaining 12 patients, tumor histopathology was low grade clear cell RCC (ccRCC) in 6, high-grade ccRCC in 3, unclassified RCC in 2, and chromophobe RCC in 1. Mean tumor size was 3.8 (range, 1.8-10.6) cm. Figs 1-3 show representative T2-weighted images, MRF T1 and T2 maps obtained for one low-grade, one high-grade, and one unclassified RCC. Histopathology of the mass are also presented for both the low-grade and high-grade cases, showing the difference in cellular and nuclear pleomorphism.Fig 4 shows the mean T1 and T2 values for two different RCC groups. Since unclassified RCC mostly presents as high-grade tumors with poor outcomes, it was combined with high-grade RCC and compared with the chromophobe/low-grade RCC group. Significant lower T2 values were obtained in the high-grade/unclassified RCC group (55±19 vs. 90±24 msec; P < 0.05). However, no significant difference was observed in T1 values between the two groups (P > 0.05).
Further statistical analysis demonstrated that MRF T1 and T2 measurements provide complementary information in characterization of RCC tumor grades (Fig 5). Using a support vector machine (SVM) with leave-one-out cross validation followed by bootstrap samples for model validation, the sensitivity and specificity for the differentiation of chromophobe/low-grade from high-grade/unclassified RCC were 89% and 98%, respectively, demonstrating the strength of multi-parametric imaging in effectively separating the two RCC groups.
Discussion and Conclusion
In this study, we evaluated the application of MRF in differentiation of RCC tumor grades. Significant difference in T2 values was noticed between low- and high-grade RCCs, matching the literature findings (4). However, no significant difference in T1 was observed, which is contrary to a recent study performed on 30 RCC subjects (3). On the other hand, a SVM-based predictive model demonstrates the strength of multi-parametric imaging in RCC characterization. Sensitivity and specificity for the differentiation of chromophobe/low-grade from high-grade/unclassified RCC were 89% and 98%. All MRF-derived quantitative maps are co-aligned which further facilitates direct comparison across different tissue properties with improved accuracy. Future work will be focused on expansion of tissue properties measured with kidney MRF, including T2* and fat fraction quantification (6). The developed approach has great potential in eliminating unnecessary biopsy/surgery in eligible patients with low-grade RCCs and provide guidance to determine the most appropriate treatment strategy.Acknowledgements
No acknowledgement found.References
1. Siegel RL. et al. CA Cancer J Clin, 2021:7-33.
2. Kale HP. et al. Urol Oncol, 2016; 433.
3. Adams LC, et al. Invest Radiol, 2019; 118-128.
4. Adams LC, et al. Cancer Imaging, 2019; 1-11.
5. MacAskil CJ, et al. Radiology, 2021; 380-387.
6. Huang S. et al. Proceedings of ISMRM, 2021; p478.
Figures