0472
Long-term effects of stimulant treatment on regional cortical thickness development in ADHD1Department of Radiology & Nuclear Medicine, Amsterdam UMC, University of Amsterdam, Amsterdam, Netherlands, 2Department of Psychology, Center for Lifespan Changes in Brain and Cognition, University of Oslo, Oslo, Norway, 3Departments of Radiology and Nuclear Medicine, Oslo University, Oslo, Norway, 4Dutch Autism and ADHD Research Center, Amsterdam Brain and Cognition, University of Amsterdam, Amsterdam, Netherlands
Synopsis
Stimulant medication is commonly used in treatment for attention-deficit/hyperactivity disorder, yet its effects on brain development remain unclear. This study investigated the long-term age-dependent effects of stimulants on cortical development after a 4-year naturalistic follow-up of adolescents and adults with ADHD using T1-weighted scans. Medication use was higher in adolescents than adults, and ADHD symptoms improved in both age groups. In line with literature on cortical development, analyses revealed reductions in apparent cortical thickness in adolescents only. However, we observed no effect of medication use on change in cortical thickness, suggesting previously identified psychostimulant effects may be transient.
Introduction
Stimulants, such as methylphenidate (MPH) and dexamphetamine (DEX), are the primary pharmacological treatment for attention-deficit/hyperactivity disorder (ADHD). They are commonly prescribed to children and adolescents with ADHD, often for extended periods of time. However, the influence of stimulants on brain development remains to be elucidated.Therefore, we conducted a randomized controlled trial (RCT) to investigate the age-dependency of the effects of MPH on the developing brain (ePOD-MPH), in which stimulant treatment-naïve children and adults with ADHD received 4-month treatment with MPH or placebo. One week after trial end, reduced apparent cortical thinning was observed in specific cortical regions of MPH-treated children, but not in adults or the placebo condition, suggesting short-term effects of MPH on brain maturation.1 Animal studies suggest that MPH administration during development may have long-term effects, which are fully expressed close to adulthood.2 Therefore, the present study aimed to investigate the long-term age-dependent effects of stimulant treatment on cortical development using a naturalistic follow-up of the initial ePOD-MPH RCT participants.
Methods
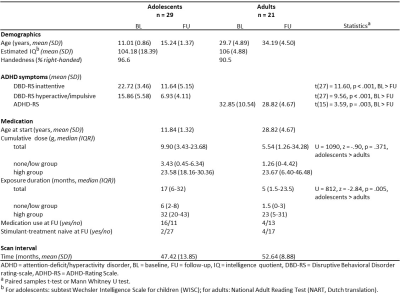
In the initial ePOD-MPH RCT, 50 boys (mean age=11.3yrs) and 49 men (mean age=28.5yrs) with ADHD were included. All participants were stimulant treatment-naïve at baseline and met strict criteria for ADHD diagnosis (all subtypes) according to the DSM-IV criteria.3 From the initial ePOD-MPH RCT, 34 adolescent and 25 adult participants returned for the 4-year follow-up assessment.ADHD symptom severity at baseline and 4-year follow-up was assessed using the Disruptive Behavioral Disorder rating-scale (DBD-RS4, adolescents) and ADHD-Rating Scale (ADHD-RS5,6, adults).
During the initial ePOD-MPH RCT, medication dosages were titrated per individual according to Dutch clinical treatment guidelines. Stimulant medication use per participant was calculated based on medication received during the ePOD-MPH RCT and medication history information between the ePOD-MPH RCT and 4-year follow-up assessment obtained from pharmacies. Cumulative dose (CD, mg) of stimulant medication was converted to MPH-equivalents as previously described.7,8 In addition, exposure duration (ED, months) to stimulant medication was calculated, with a 30-day permissible gap to allow for commonly occurring “medication holidays”.
A high resolution 3D T1-weighted fast-field echo sequence (TR/TE/FA=9.8/4.6ms/8°, voxel size=0.875x0.875x1.2mm, slices=120, reconstruction matrix=256) was acquired at baseline and 4-year follow-up assessment with an 8-channel receive-only head coil using 3T Philips Intera, Achieva and Ingenia MR Scanners (Philips Medical Systems, Best, The Netherlands).
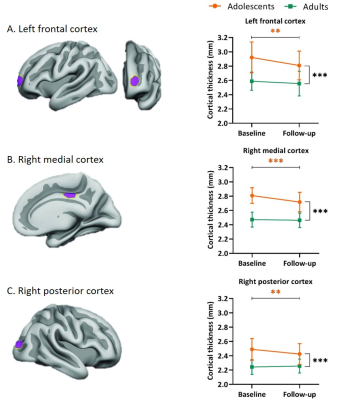
Data processing and selected regions of interest (ROIs) were equivalent to the initial ePOD-MPH RCT.1 Vertex-wise cortical thickness was estimated across the brain surface using the FreeSurfer longitudinal processing stream (v5.3).9-13 Predefined ROIs (Figure 2) in the left frontal, right medial and right posterior cortices, were selected based on prior findings of psychostimulant effects on cortical thickness by Shaw et al.1,14 MNI coordinates of the vertices corresponding to peak group differences were converted to the Talairach coordinate system, and dilated 15 times to create hexagonal ROIs of 550 mm2 when transformed to subjects’ brain surfaces.
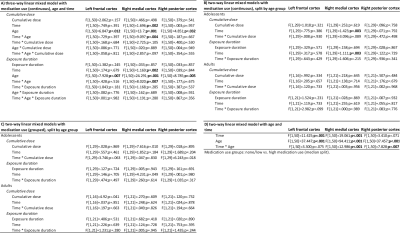
Analyses were performed in SPSS v.26 (IBM). CD and ED were log-transformed to allow for parametric statistics. Effects of medication use (CD/ED), age group (adolescents/adults) and time (BL/FU) on change in cortical thickness were assessed using three-way linear mixed models, as well as split by age group. Medication use variables were included as 1) continuous, and 2) grouped (none/low vs. high; median split) variables. All analyses were corrected for multiple comparisons (FDR=5%).
Results
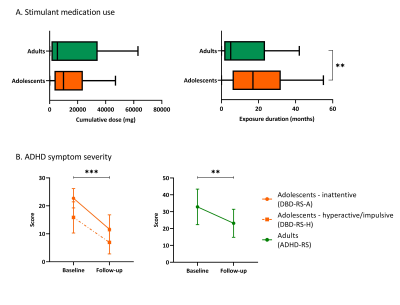
From the participants that returned for the follow-up assessment, 9 participants (5 adolescents, 4 adults) were excluded from analysis due to incomplete data, undisclosed prior MPH use and/or structural brain abnormalities. Sample characteristics of the remaining participants are shown in Table 1. ED was significantly higher in adolescents than adults (p=.005) and ADHD symptom severity was significantly reduced at follow-up compared to baseline in both age groups (adolescents: inattentive p<.001, hyperactive/impulsive p<.001; adults: p=.003; Table 1, Figure 1). We observed no significant age-dependent effects of medication use on change in regional cortical thickness (three-way interaction: age*time*CD and age*time*ED; Table 2A) or when split by age-group (two-way interaction: time*CD and time*ED, in adolescents and adults; Table 2B). This was confirmed by analysis per age group with medication use as grouped variables (two-way interaction: time*grouped CD and time*grouped ED, in adolescents and adults; Table 2C). Two-way mixed model analysis without medication use revealed an age-by-time interaction effect on right medial (p<.001) and posterior (p=.007) cortical thickness. Moreover, we observed a main effect of age (p<.001) and time (p=.001) on left frontal cortical thickness (Table 2D). Indeed, post-hoc analysis per age group revealed that cortical thickness significantly decreased in all ROIs in adolescents only (left frontal cortex p=.003, right medial cortex p<.001, right posterior cortex p=.004, Figure 2).Discussion
In contrast to our hypothesis, we found no evidence for long-term effects of stimulant treatment on regional cortical thickness in adolescents or adults with ADHD, suggesting that previously identified age-dependent effects of MPH on cortical thickness may be transient. Our findings of apparent cortical thinning in adolescents, but not in adults, are in line with literature on cortical development.15,16 Future studies should include prescription adherence measures to better reflect true medication intake during naturalistic follow-up. Moreover, comparison with age-matched typically-developing volunteers is needed to further investigate and interpret the effects of stimulant treatment during development.Acknowledgements
This study was funded by the Dutch non-profit organizations KiddyGoodPills and Suffigium. The ePOD-MPH RCT (baseline data) was funded by a personal research grant awarded to LR by the Academic Medical Center, University of Amsterdam, and 11.32050.26ERA-NET PRIOMEDCHILD FP 6 (EU). We would like to thank all participants and their parents for their contribution to this study and all students that helped with collection and analysis of the data.References
1. Walhovd, K.B., Amlien, I., Schrantee, A., Rohani, D.A., Groote, I., Bjørnerud, A., Fjell, A.M., Reneman, L., 2020. Methylphenidate effects on cortical thickness in children and adults with attention-deficit/hyperactivity disorder: A randomized clinical trial. American Journal of Neuroradiology 41, 758–765. doi:10.3174/ajnr.a6560
2. Andersen, S.L., Navalta, C.P., 2004. Altering the course of Neurodevelopment: A framework for understanding the enduring effects of psychotropic drugs. International Journal of Developmental Neuroscience 22, 423–440. doi:10.1016/j.ijdevneu.2004.06.002
3. American Psychiatric Association, 1994. Diagnostic and Statistical Manual of Mental Health Disorders. 4th. Washington, DC: American Psychiatric Association
4. Pelham WE Jr, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry. 1992 Mar;31(2):210-8. doi: 10.1097/00004583-199203000-00006. Erratum in: J Am Acad Child Adolesc Psychiatry 1992 Nov;31(6):1177. PMID: 1564021.
5. Kooij, J. (2012). Adult ADHD: Diagnostic assessment and treatment. London: Springer-Verlag.
6. Sandra Kooij JJ, Marije Boonstra A, Swinkels SH, Bekker EM, de Noord I, Buitelaar JK. Reliability, validity, and utility of instruments for self-report and informant report concerning symptoms of ADHD in adult patients. J Atten Disord. 2008 Jan;11(4):445-58. doi: 10.1177/1087054707299367. PMID: 18083961.
7. Norman LJ, Sudre G, Bouyssi-Kobar M, Sharp W, Shaw P. A Longitudinal Study of Resting-State Connectivity and Response to Psychostimulant Treatment in ADHD. Am J Psychiatry. 2021 Aug 1;178(8):744-751. doi: 10.1176/appi.ajp.2021.20091342. Epub 2021 Jun 4. PMID: 34086483; PMCID: PMC8528221.
8. Nasky KM, 2018. Stimulant Dose Conversion Calculator. https://psychopharmacopeia.com/stimulant_conversion.php
9. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999 Feb;9(2):195-207. doi: 10.1006/nimg.1998.0396. PMID: 9931269.
10. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000 Sep 26;97(20):11050-5. doi: 10.1073/pnas.200033797. PMID: 10984517; PMCID: PMC27146.
11. Dale AM, Sereno MI. Improved Localizadon of Cortical Activity by Combining EEG and MEG with MRI Cortical Surface Reconstruction: A Linear Approach. J Cogn Neurosci. 1993 Spring;5(2):162-76. doi: 10.1162/jocn.1993.5.2.162. PMID: 23972151.
12. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999 Feb;9(2):179-94. doi: 10.1006/nimg.1998.0395. PMID: 9931268.
13. Ségonne F, Grimson E, Fischl B. A genetic algorithm for the topology correction of cortical surfaces. Inf Process Med Imaging. 2005;19:393-405. doi: 10.1007/11505730_33. PMID: 17354712.
14. Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, Evans AC, Rapoport JL. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009 Jan;166(1):58-63. doi: 10.1176/appi.ajp.2008.08050781. Epub 2008 Sep 15. PMID: 18794206; PMCID: PMC2700349.
15. Tamnes, C.K., Østby, Y., Fjell, A.M., Westlye, L.T., Due-Tønnessen, P., Walhovd, K.B., 2009. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex 20, 534–548. doi:10.1093/cercor/bhp118
16. Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008 Apr 2;28(14):3586-94. doi: 10.1523/JNEUROSCI.5309-07.2008. PMID: 18385317; PMCID: PMC6671079.
Figures