0470
Effect of Intravoxel Incoherent Motion on NODDI Diffusion Parameters in both Healthy and Psychotic Spectrum Disorder Populations1Radiology, New York University School of Medicine, New York, NY, United States
Synopsis
We investigated the intravoxel incoherent imaging (IVIM) effect on neurite orientation dispersion and density imaging (NODDI) metric calculations in both healthy controls (N=36) and a clinical population of individuals with a psychotic spectrum disorder (PSD) (N=55) by extending the original model to include a microvascular blood compartment. We found that without controlling for the IVIM effect, several NODDI metrics were possibly misestimated, which subsequently affected group comparisons and results.
Background
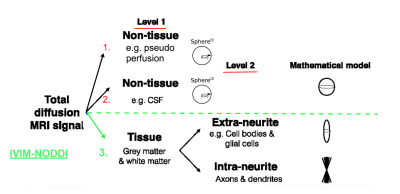
The intravoxel incoherent motion (IVIM) method is a promising noninvasive technique that has been successfully applied to capture brain perfusion pathologies in several brain disorders. Previous work has shown that extending diffusion tensor and free-water models to incorporate IVIM both a) quantifies perfusion, and b) more accurately estimates both the tissue and free-water compartments1,2. The IVIM-FWI model is especially promising as it aims to disentangle isotropic free water, thought to describe the extracellular compartment, and perfusion (Figure 1). The estimation of the perfusion fraction (PF), which describes the relative fraction of the microvascular compartment, and FW may be affected when the effects of the other are not taken into consideration2,3. Previous histological studies have suggested that distinct microvascular and microstructural properties may impact PF (capillary density, neurovascular unit cells and the blood brain barrier) and FW metrics (inflammation and atrophy), which can be obscured if metrics are not separated4. Despite its relevance to PSD and neurodegenerative disorder fields1,2, the free-water model description of the tissue compartment using a single tensor is non-specific. Therefore, we propose to evaluate, for the first time, the inclusion of an IVIM compartment within the neurite orientation dispersion and density imaging (NODDI), a model which includes intra-neurite, extra-neurite, as well as free-water compartments. We specifically evaluated how IVIM affects: 1) the NODDI metrics estimation in HC and a clinical population (psychotic spectrum disorders( PSD)) and 2) the group comparisons between HC and PSD. This second analysis aims to provide insight into the effects of neglecting the IVIM effect in dMRI disease examinations and therefore our understanding of the biological bases of neuropathology.Methods
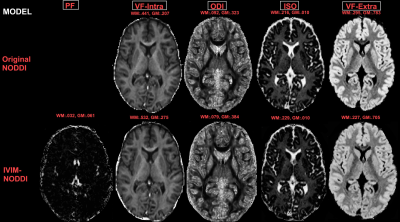
Simulations were conducted to test the IVIM-NODDI model and examine the contamination of the NODDI parameters by the perfusion in the original NODDI model by varying the: a) tissue fraction; b) acquisition scheme, and c) perfusion fraction as previously described2. Anatomical and diffusion images were acquired for 55 PSD patients (16 schizophrenia (SZ), 20 schizoaffective (SZA), 18 bipolar with psychotic features (BPF)), and 36 healthy comparison controls (HC) 18-31 years old using a 3T Prisma MRI scanner. Isotropic 1.5mm diffusion images with b-values between 0 and 3000 s/mm2 were obtained. The models and their fitting were implemented using DMIPY software2,5. Separate NODDI and NODDI-IVIM models were applied to gray and white matter using previously reported optimal parallel diffusivity6. The dMRI metrics calculated were: the orientation dispersion index (ODI), intra-neurite volume fraction (VF-Intra), isotropic volume fraction (ISO/FW), extra-neurite volume fraction (VF-Extra) and PF (Figure 2). Mean gray and white matter metrics were calculated bilaterally for 34 cortical regions of interest (ROI) delineated by the Desikan-Killiany atlas7. Since including age, sex, pre-morbid IQ, or cortical thickness as covariates did not affect the results, independent t-test analyses were used here to first evaluate differences between NODDI and NODDI-IVIM metrics, and second, compare how NODDI with and without an IVIM compartment affected between-group comparisons between PSD, PSD subtypes and HCs in each ROI. The Benjamini-Hochberg (BH) procedure was employed in each analysis to correct for multiple comparisons and decrease the false discovery rate (FDR)8. Differences were considered significant for q < .05 BH FDR and at trend-level for p < .05.Results
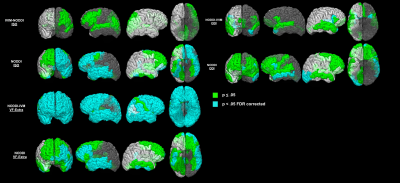
Simulations showed that regardless of acquisition scheme and tissue fraction percent, increased perfusion contribution resulted in overestimation of FW/ISO and underestimation of the diffusivity of tissue compartment for the original NODDI model. In the brain data, we found that the NODDI without IVIM calculated:1) higher ISO in gray and white matter; 2) higher VF-Extra in gray matter, and 3) lower VF-Intra in white matter, compared to NODDI-IVIM (Figure 3). When comparing group differences between PSD, and PSD subtype groups (SSD, SZA, SZ, BPF) and HC groups we found that the new NODDI-IVIM model showed 1) more group differences in ODI, VF-Intra and VF-Extra in PSD and all PSD sub-types, and 2) less ISO group differences in PSD and all PSD sub-types (Figure 4).Discussion
The FW/ISO and VF-Extra metrics derived from FWI and NODDI are thought to provide important information about extracellular processes such as atrophy, inflammation, and extra-neurites (e.g., cell bodies and glial cells) in both clinical and healthy populations. Here, we demonstrate that without accounting for the IVIM effect, the NODDI derived ISO/FW may be misestimated which affects the examination of the PSD vs HC group differences. The proposed model may disentangle the perfusion effect from that of free water, and alongside a good acquisition and model fit, distinguish between changes that originate from the capillary blood perfusion versus extracellular space to allow for more specific characterization of pathology important for understanding and classifying disorders.Conclusion
We demonstrate that the NODDI model with an IVIM compartment may be able to separate out PF vs FW/ISO effects and may more specifically characterize microstructural and microvascular pathologies. This model may provide useful, and more accurate, biomarkers of abnormal vasculature and microstructure and help elucidate neuropathology in psychiatric and neurological disorders.Acknowledgements
No acknowledgement found.References
1.Le Bihan, D. et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology (1988).
2. Rydhög, A. S. et al. Separating blood and water: Perfusion and free water elimination from diffusion MRI in the human brain. Neuroimage 156, (2017).
3. Vieni, C. et al. Effect of intravoxel incoherent motion on diffusion parameters in normal brain. Neuroimage (2020).
4. Kealy, J., Greene, C. & Campbell, M. Blood-brain barrier regulation in psychiatric disorders. Neuroscience Letters (2020).
5. Fick, R. H. J., Wassermann, D. & Deriche, R. The Dmipy Toolbox: Diffusion MRI Multi-Compartment Modeling and Microstructure Recovery Made Easy. Front. Neuroinform. 13, 64 (2019).
6. Guerrero, J. M. et al. Optimizing the intrinsic parallel diffusivity in NODDI: An extensive empirical evaluation. PLoS One (2019).
7. Desikan, R. S. et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980 (2006).
8. Hochberg, B. Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 57, 289–300 (1995).
Figures