0466
Alterations in Temporal Lobe Functional Connectivity Differ Based on Clinical Outcomes in Patients with Mesial Temporal Sclerosis1Thomas Jefferson University, Philadelphia, PA, United States, 2The University of New Mexico, Albuquerque, NM, United States
Synopsis
Mesial temporal sclerosis (MTS) is a severe form of temporal lobe epilepsy. Resection is the treatment of choice for refractory MTS but can still show seizure recurrence. We used seed-to-voxel analysis via amplitude synchronization to determine alterations in functional connectivity in MTS based on seizure-free status one year post-resection. Positive and negative correlations between temporal lobe structures and other brain regions were calculated and compared. We found changes in connectivity in MTS between ascending reticular activating system structures such as the thalamus and brainstem and temporal lobe structures. We also found differences in connectivity alterations between seizure-free and non-seizure-free patients.
Introduction
Epilepsy is a disease of abnormal brain networks. Temporal lobe epilepsy (TLE) is the most common form of refractory focal epilepsy1,2. Mesial temporal sclerosis (MTS) is a form of TLE and can cause severe complications including memory deficits and cognitive impairment3. Estimates of the prevalence and incidence of medically refractory MTS in the U.S. range from 0.51 – 0.66 cases per 1,000 people and 3.1 – 3.4 cases per 100,000 people per year, respectively, showing a significant burden of disease4.Surgical resection is the treatment of choice for refractory epilepsy and often yields effective results5. Methods to identify areas for resection are still improving6. Currently, there is not much research comparing MTS patients and control connectivity using seed-to-voxel analysis via amplitude synchronization. Elucidating noninvasive imaging biomarkers for abnormal connections could identify different subtypes of MTS and determine areas for surgical resection6.
In this study, we utilize patient rsfMRI data to identify differences in functional connectivity between temporal lobe regions and other brain regions in refractory MTS patients and controls. Uniquely, we examine temporal lobe connectivity based on its sub-segmentation into mesial and lateral sections, providing more insight into connectivity differences in regions relevant to MTS pathogenesis, anatomy, and surgery. We further analyze connectivity differences between patients who remained seizure-free at 12 months post-resection (SF) and those who did not (NSF).
Methods
This is a retrospective study of preoperative rsfMRI data from 31 MTS patients (age 22-69) and 16 controls (age 21-35). fMRI data were preprocessed and registered to a standard space. Using each subject's T1 scan, medial and lateral temporal regions were automatically segmented, manually revised and then fit to a standard space using a symmetric normalization (SyN) registration algorithm. For each subject, we used dual regression analysis to detect amplitude synchronization of medial and lateral temporal segments with the rest of the brain. We calculated the overlapped volume ratio of synchronized voxels within target regions including the thalamus (total and bilateral), insula (bilateral), cerebellum (bilateral), brainstem, total gray matter, medial and lateral gyri (bilateral) and supratentorial (bilateral). A general linear model was used and data were regressed for covariates of epilepsy duration and patient age at scan. Bonferroni correction was applied. Data was analyzed based on patient seizure-free status at one year.Results
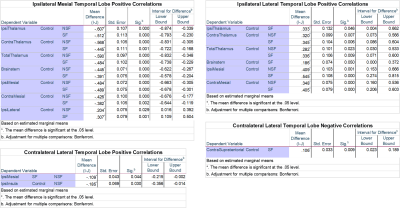
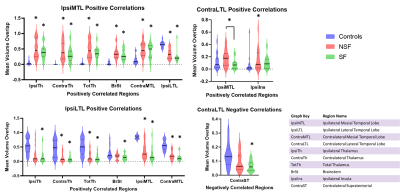
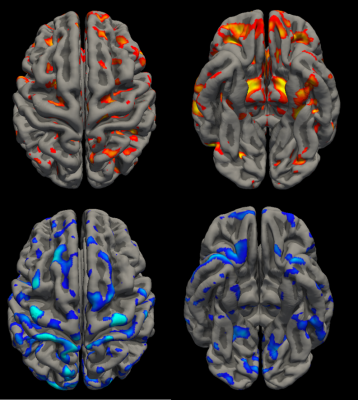
Significant results can be seen as values in Figure 1 and violin plots in Figure 2. An example of temporal lobe correlations for one patient can be seen in Figure 3. SF and NSF MTS patients showed increased positive correlations compared to controls between the mesial temporal lobe ipsilateral to the seizure focus and the ipsilateral thalamus, contralateral thalamus, total thalamus, brainstem, and contralateral mesial temporal lobe, while decreased positive correlations were seen with the ipsilateral lateral temporal lobe. Both SF and NSF patients showed increases in positive correlations compared to controls between the lateral temporal lobe ipsilateral to the seizure focus and the contralateral thalamus, total thalamus, the ipsilateral mesial temporal lobe, and the contralateral mesial temporal lobe. Decreases in positive correlations compared to controls were seen between the ipsilateral lateral temporal lobe and the ipsilateral thalamus, the contralateral thalamus, the total thalamus, the brainstem, the ipsilateral mesial temporal lobe, and the contralateral mesial temporal lobe. Decreases in positive correlations from the contralateral lateral temporal lobe were also seen in the ipsilateral temporal lobe in SF patients compared to NSF and the ipsilateral insula in controls compared to NSF. Decreased negative correlations were seen in SF patients compared to controls between the contralateral lateral temporal lobe and the contralateral supratentorial.Discussion
Our results demonstrated increased functional connectivity between the mesial temporal lobe ipsilateral to the seizure focus and regions that are part of the ascending reticular activating system (ARAS), such as the thalamus and brainstem. Meanwhile, the ipsilateral lateral temporal lobe showed decreased functional connectivity to the same structures in MTS patients. While prior literature has reported overall decreased connections between cortical structures and the ARAS in MTS7, our analysis finds that differences in connectivity may exist in the mesial and lateral lobes, with the mesial showing increased connectivity. Furthermore, SF patients appeared to have decreased connectivity between temporal lobe structures and select other brain regions. These changes indicate differences in connectivity compared to controls that are not seen in NSF patients and may provide insight into the pathological mechanisms underlying altered brain networks that may help determine SF status.Conclusion
Our analysis showed significant differences between connectivity patterns in refractory MTS patients based on their seizure-free status at one year. Along with some significant differences directly between SF and NSF groups, many structures showed altered connectivity in SF but not NSF patients, or in NSF but not SF patients, compared to controls. These findings indicate that seed-to-voxel analysis via amplitude synchronization can detect pathological changes in functional connectivity in MTS patients as well as implicate changes that may determine seizure-free status after resection.Acknowledgements
No acknowledgement found.References
1. Allone C, Lo Buono V, Corallo F, Pisani LR, Pollicino P, Bramanti P, et al. Neuroimaging and cognitive functions in temporal lobe epilepsy: A review of the literature. J Neurol Sci. 2017 Oct 15;381:7–15.
2. Wu C, Jermakowicz WJ, Chakravorti S, Cajigas I, Sharan AD, Jagid JR, et al. Effects of surgical targeting in laser interstitial thermal therapy for mesial temporal lobe epilepsy: A multicenter study of 234 patients. Epilepsia. 2019 May 21;60(6):1171–1183.
3. Tramoni-Negre E, Lambert I, Bartolomei F, Felician O. Long-term memory deficits in temporal lobe epilepsy. Rev Neurol. 2017 Aug 31;173(7-8):490–497.
4. Asadi-Pooya AA, Stewart GR, Abrams DJ, Sharan A. Prevalence and Incidence of Drug-Resistant Mesial Temporal Lobe Epilepsy in the United States. World Neurosurg. 2017 Mar;99:662–666.
5. Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012 Mar 7;307(9):922–930.
6. Winston GP. The role of magnetic resonance imaging techniques in the diagnosis, surgical treatment and biological understanding of epilepsy. Quant Imaging Med Surg. 2015 Apr;5(2):186–187.
7. Englot DJ, D’Haese P-F, Konrad PE, Jacobs ML, Gore JC, Abou-Khalil BW, et al. Functional connectivity disturbances of the ascending reticular activating system in temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2017 Nov;88(11):925–932.
Figures