0464
Microscopic fractional anisotropy may be sensitive to unilateral hippocampal abnormalities in temporal lobe epilepsy1Medical Biophysics, Western University, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Epilepsy Program, Clinical Neurological Sciences, Western University, London, ON, Canada, 4School of Biomedical Engineering, Western University, London, ON, Canada
Synopsis
Drug-resistant temporal lobe epilepsy (TLE) can often be treated surgically, but successful intervention depends on the use of imaging to localize the epileptic focus. Here, microscopic fractional anisotropy (μFA), fractional anisotropy (FA), and mean diffusivity (MD) were investigated to assess their sensitivities to abnormalities in TLE. μFA was significantly reduced in the ipsilateral hippocampal subfield containing the cornu ammonis 3/4 and dentate gyrus (p=0.0044), suggesting that it may be sensitive to pathological hippocampal abnormalities. Furthermore, differences between ipsilateral and contralateral μFA and MD, but not FA, correlated with ipsilateral atrophy in that same region.
Introduction
Temporal lobe epilepsy (TLE) is the most common form of focal epilepsy in adults. While many epilepsy patients are able manage their seizures with medication, more than 30% of cases of focal epilepsy are drug-resistant 1. Unilateral temporal lobectomy can grant seizure freedom to many patients with drug-resistant TLE, but the procedure's success depends on pre-surgical localization of the seizure focus 2. Patients with visible unilateral mesial temporal sclerosis (MTS), which manifests as a T2 signal abnormality or reduction in hippocampal volume in MRI, generally have better surgical outcomes than MRI-negative patients (75% vs 51% achieve seizure freedom) 3, demonstrating the need for improved imaging techniques for seizure focus localization.Microscopic fractional anisotropy (μFA) is a diffusion MRI (dMRI) metric that estimates water diffusion anisotropy independent of neuron fiber orientation, potentially giving it high specificity to microstructural abnormalities in neural tissue 4. In this preliminary study, the ability of μFA to detect hippocampal abnormalities in TLE patients was investigated and compared against those of the more commonly used fractional anisotropy (FA) and mean diffusivity (MD) dMRI metrics.
Methods
Twelve patients with drug-resistant TLE, and presumed to have unilateral seizure foci based on standard radiological assessments, were scanned at 3T using T1-MPRAGE, DTI, and μFA protocols. The study was performed with appropriate Research Ethics Board approval and with informed consent. The T1 scan had 0.8x0.8x0.8mm resolution and TE/TR=2.3/2400ms. The DTI scan had 1.6x1.6x1.6mm resolution, TE/TR=99/5500ms, and consisted of 6, 36, and 60 linear tensor encoded (LTE) diffusion-weighted volumes at b-values of 0, 1000, and 2000s/mm2. The μFA protocol had 1.8x1.8x1.8mm resolution, TE/TR=92/4900ms, and consisted of 8 LTE scans at b=2000s/mm2 and 3, 6, and 16 spherical tensor encoded scans at b=100, 1000, and 2000s/mm2. Three patients underwent anterior temporal lobectomy surgery and were confirmed to have MTS; they are herein referred to as the definite MTS subgroup and the other nine patients are referred to as the presumed MTS subgroup.For each patient, μFA was estimated by fitting the μFA protocol data to the linear regression model described by Arezza et al 5. FA and MD were estimated by fitting the subset of the DTI data from b=0-1000s/mm2 to the DTI model. The μFA, FA, and MD volumes were registered to T1-space, and a deep-learning surface-based unfolding pipeline (Hippunfold 6,7) was used to segment the hippocampus in the T1-MPRAGE volume into subiculum, cornu ammonis (CA) 1-4, and dentate gyrus subfields. Smaller labels were combined to form three distinct regions: the subiculum (SB), CA1+2 (CA1/2), and CA3+4 plus dentate gyrus (CA3/4).
Results
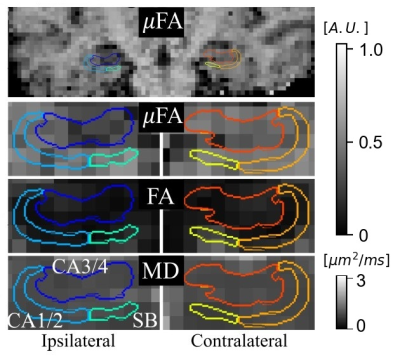
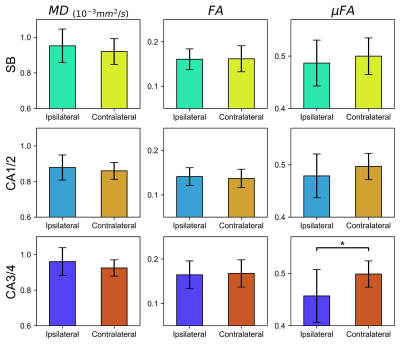
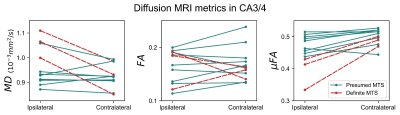
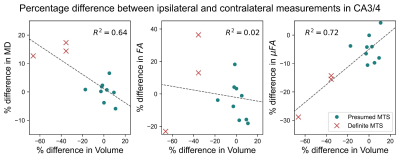
Example μFA, FA, and MD slices from one of the TLE patients from the presumed MTS subgroup are depicted in Fig. 1. Comparisons between dMRI metrics in the ipsilateral and contralateral sides of the hippocampus across all twelve patients revealed that only μFA in CA3/4 was statistically significant; the mean ipsilateral μFA was observed to be lower than contralateral uFA in the region (p=0.0044) (Fig. 2).Interpatient comparisons in CA3/4 (Fig. 3) revealed that ipsilateral μFA was reduced compared to contralateral μFA in 11/12 patients (92% of the group), while ipsilateral FA was reduced in 7/12 patients (58%) and ipsilateral MD was elevated in 10/12 (83%) (Fig. 3). Percentage difference (%Δ) between ipsilateral and contralateral μFA and MD both correlated with %Δ in CA3/4 volume (r2=0.72 and 0.64, respectively), but %ΔFA did not (r2=0.02) (Fig. 4). In CA3/4, mean %Δvolume, %ΔμFA, %ΔFA, and %ΔMD were -11.0%, -8.5%, -0.9%, and +3.9%, respectively.
Discussion
Of the dMRI metrics examined in this preliminary work, μFA was observed to be the most sensitive to hippocampal abnormalities in TLE. The difference between ipsilateral and contralateral μFA in CA3/4 was the only statistically significant result amongst the dMRI metrics across all regions, and the magnitude of this difference (8.5%) was greater than those of MD (3.9%) and FA (0.9%) in the same region. Furthermore, in CA3/4, %ΔμFA strongly correlated with %Δvolume, which is a known marker of MTS. %ΔMD also correlated with %Δvolume in CA3/4 while no correlation was observed between %ΔFA and %Δvolume.The correlation between %ΔμFA and %Δvolume in CA3/4 can be attributed to the fact that hippocampal atrophy likely results from neurodegenerative processes that also alter microstructure, such as demyelination and axonal degradation. FA may be less specific to these abnormalities than μFA due to confounding factors such as its dependence on the orientation of diffusion compartments, given that crossing neuron fibers are present throughout the hippocampus.
In summary, this preliminary work demonstrated that μFA may be sensitive to hippocampal abnormalities that occur in unilateral TLE. Future work should aim to acquire μFA protocols at higher resolution to reduce the impact of partial volume effects on the measurements, and to increase the number of acquisitions in the protocol to improve SNR for further investigation into μFA’s potential use as a clinical biomarker for seizure focus localization in TLE.
Acknowledgements
Canada First Research Excellence Fund to BrainsCAN, Canada Research Chairs Program, Natural Sciences and Engineering Research Council of Canada
Dr. Jorge Burneo is the Jack Cowin endowed Chair in Epilepsy Research at Western University.
References
1. Kwan, P. & Brodie, M. J. Early identification of refractory epilepsy. N. Engl. J. Med. 342, 314–319 (2000).
2. Blumcke, I. et al. Histopathological Findings in Brain Tissue Obtained during Epilepsy Surgery. N. Engl. J. Med. 377, 1648–1656 (2017).
3. Muhlhofer, W., Tan, Y.-L., Mueller, S. G. & Knowlton, R. MRI-negative temporal lobe epilepsy-What do we know? Epilepsia 58, 727–742 (2017).
4. Shemesh, N. et al. Conventions and nomenclature for double diffusion encoding NMR and MRI. Magn. Reson. Med. 75, 82–87 (2016).
5. Arezza, N. J. J., Tse, D. H. Y. & Baron, C. A. Rapid microscopic fractional anisotropy imaging via an optimized linear regression formulation. Magn. Reson. Imaging 80, 132–143 (2021).
6. DeKraker, J., Ferko, K. M., Lau, J. C., Köhler, S. & Khan, A. R. Unfolding the hippocampus: An intrinsic coordinate system for subfield segmentations and quantitative mapping. Neuroimage 167, 408–418 (2018).
7. Khan, A., DeKraker, J., & Haast, R. Hippunfold: Snakemake BIDS app for hippocampal unfolding & subfield segmentation (v0.3.0). Zenodo. (2021). Available: https://doi.org/10.5281/zenodo.4488922
Figures