0455
Towards frequency robust tailored and universal pulses in the human heart at 7T1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany, 2German Cancer Research Center (DKFZ), Medical Physics in Radiology, Heidelberg, Germany, 3University of Minnesota, Center for Magnetic Resonance Research, Minneapolis, MN, United States
Synopsis
This work demonstrates the design and application of frequency robust, subject-tailored or universal, non-selective kT-point pulses to achieve a homogeneous flip-angle within the 3D human heart at 7T across a 1400Hz wide frequency range that includes water and six fat frequencies. Frequency robust universal pulses were computed offline based on 31 3D B1+-maps and could be used in time-critical situations for calibration-free 3D heart flip-angle homogenization. Experimental data at 7T validates the flip-angle predictions and demonstrates successful excitation of fat and water in the human heart at 7T.
Introduction
3D imaging at ultra-high fields (UHF) in the human body and particularly in the heart is highly challenging due to the inhomogeneous transmit field (B1+) leading to spatially varying flip-angle (FA) patterns. Tailored and universal dynamic parallel transmission (pTx)1,2 have been demonstrated to sufficiently improve the FA homogeneity across the 3D heart volume at UHF.3-5 In contrast to static pTx, however, dynamic pTx can be more susceptible to deviations in the main magnetic field (∆B0), which can be substantial in 7T cardiac MRI.6,7Here, we demonstrate for the first time the feasibility of using frequency robust (fRob) tailored (TP) and calibration-free pulses (universal pulses8; UP) in the human body to achieve homogeneous flip-angle (FA) distributions throughout the heart across a 1400Hz wide frequency range that includes water and six fat frequencies. The proposed frequency robust tailored (fRob-TPs) and universal pulses (fRob-UPs) were computed offline using 31 B1+-maps at seven resonance frequencies ranging from -1129 to 178Hz.9 The fRob-TPs were successfully validated experimentally in-vivo at 7T in five volunteers.
Methods
MRI was performed at 7T (Siemens Magnetom, Germany) according to an approved IRB protocol on 36 healthy volunteers in two groups: i) library: 31 volunteers (18M/12F, 21-66years, BMI=19-35kg/m2) and ii) test-cases: 5 volunteers (4M/1F, 22-40years, BMI=20-25kg/m2) using a 32-element body coil array (MRI.TOOLS, Germany) driven in 8Tx/32Rx mode. Safety limits and coil placements were identical to previous works.3-5 Relative 3D B1+-maps of the thorax were acquired for each volunteer under shallow breathing with a radial phase-encoding (RPE) trajectory10 and were reconstructed non-respiration resolved (nominal FA=20°, TE/TR=2.02/40ms, FOV=250x312x312mm3, resolution=(4mm)3).3TPs and UPs were designed using the small-tip-angle approximation for a trait-off between RF power and FA root-mean-squared-error11 in MATLAB for the heart volume using B1+-maps from single or multiple subjects.3-5 These approaches were extended to cover multiple frequencies to achieve a homogeneous FA for all selected frequencies in the heart volume. The used source code and the B1+-maps can be downloaded from https://github.com/chaigner/FRobUP_body.
fRob-TPs were designed (4kT-points, duration=0.96ms) in less than a minute and the fRob-UPs were computed offline (2-8kT-points, duration=0.48-1.92ms) using 31 B1+-maps and up to seven different resonance frequencies in approximately 60 minutes. These seven frequencies (water and six fat peaks) were included by adding different global ∆B0-offsets of -1129Hz, -1010Hz, -772Hz, -576Hz, -115Hz, 0Hz and 178Hz.9 The performance of the pulses was analyzed using the coefficient-of-variation (CV) in the 3D heart volumes.
3D gradient-echo (GRE) scans with parameters fitting to the B1+-scans were acquired in five test cases to validate TP and fRob-TP.
Results and Discussion
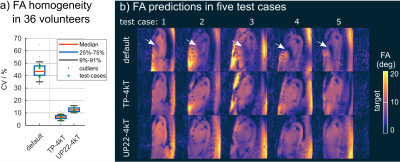
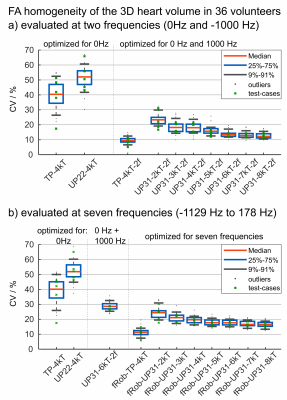
Figure 1 shows the FA evaluation of different pTx settings for one resonance frequency (water=0Hz). Across 36 B1+-maps, both TP-4kT and UP22-4kT (previously designed single-frequency-UP5 based on 22 B1+-maps) result in low CV values with a median of 6.7% and 12.5% (compared to 43.4% for a default shim setting optimized by the manufacturer based on EM-models) in the 3D heart volumes. This is also reflected by the homogeneous FA distribution across the test cases.Figure 2 shows the performance of different pTx pulses evaluated at two (a) and at seven (b) resonance frequencies. Figure 2a shows that single-frequency pulses such as TP-4kT and UP22-4kT fail to achieve homogeneous FAs which is expressed by a median CV of 40.4% and 52% across all 36 volunteers and both frequencies. Dual-frequency ("2f") pulses, in contrast, result in lower CV values of 9.4% (TP-4kT-2f) and 13.4% (UP31-6kT-2f). As expected, Figure 2b shows lower performance for single-frequency pulses across all seven frequencies. Interestingly, also UP31-4kT-2f results in elevated CV values with a median of 28.6% if evaluated at all seven frequencies. This indicates that dual-frequency design is not sufficient to ensure frequency-robust pulses (see Figure 4). Robustness across the frequency range was only achieved with pulses optimized for all seven frequencies yielding median CV values of 11.2% (fRob-TP-4kT) and 17.6% (fRob-UP31-6kT).
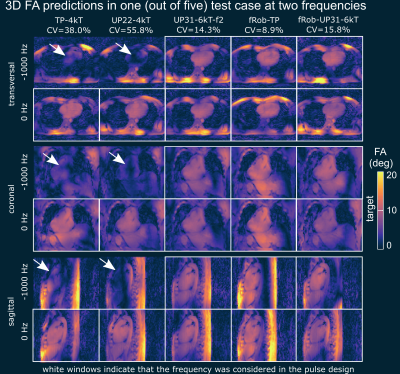
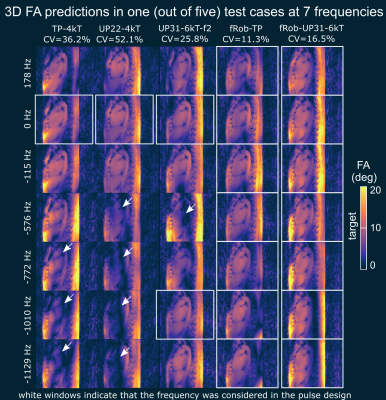
Figures 3 and 4 show the quantitative median CV values across the 3D heart volume and qualitative comparison of the 3D FA predictions for different pulses evaluated at two (Figure 3) and seven (Figure 4) frequencies. The major FA variations and dropouts demonstrate the need to consider multiple frequencies in the pulse design if water and fat should be acquired at once. The resulting FA predictions of fRob-TP-4kT and fRob-UP31-6kT show improved frequency robustness compared to the other pulses and demonstrate that a homogeneous FA can be expected for all resonance frequencies. Similar predictions are obtained in all five test cases.
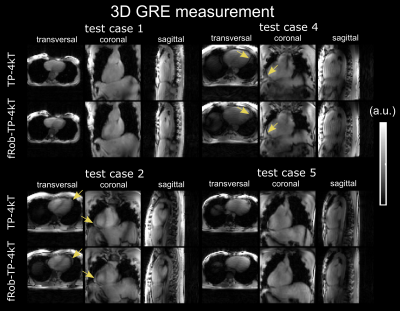
Figure 5 shows the acquired free-breathing 3D GRE images using TP-4kT and fRob-TP-4kT. fRob-UP31-6kT still needs to be validated experimentally, which was not yet possible due to time constraints. In volunteers that contain fatty tissue around the heart (indicated by arrows) fRob-TP-4kT results in a more homogeneous signal. This demonstrates that frequency robust pulses could be used to acquire data at multiple resonance frequencies as it is required for 3D fat/water imaging.6
Conclusion
This study demonstrates the benefits of fRob-TP and fRob-UP in the human body at 7T to acquire homogeneous signals from multiple resonance frequencies. Moreover, fRob-UP could be used in time-critical situations for calibration-free 3D heart FA homogenization that could push quantitative 3D imaging in the body at 7T.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation SCHM 2677/2-1 and GRK2260, BIOQIC.References
1) Padormo, F., Beqiri, A., Hajnal, J. V., and Malik, S. J. (2016), Parallel transmission for ultrahigh‐field imaging. NMR in Biomed., 29: 1145– 1161. doi: 10.1002/nbm.3313
2) Gras, V., Vignaud, A., Amadon, A., Le Bihan, D., and Boulant, N. (2017), Universal pulses: A new concept for calibration‐free parallel transmission. Magn. Reson. Med., 77: 635-643. doi:10.1002/mrm.26148
3) Dietrich, S., Aigner, C.S., and Schmitter, S. (2020), 3D Free-breathing Multi-channel absolute B1+ Mapping in the Human Body at 7T, Magn. Reson. Med., doi:10.1002/mrm.28602
4) Aigner, C.S., Dietrich, S., and Schmitter, S. (2020), Three-dimensional static and dynamic parallel transmission of the human heart at 7T, NMR in Biomed., doi:10.1002/nbm.4450
5) Aigner, C.S., Dietrich, S., Schaeffter, T, and Schmitter, S. (2021), Calibration-free pTx of the human heart at 7T via 3D universal pulses. Magn Reson Med. 00: 1– 15. https://doi.org/10.1002/mrm.28952
6) Dietrich, S., Mayer, J., Aigner, C.S., Kolbitsch, C., Schulz-Menger, J., Schaeffter, T., and Schmitter S. Respiratory resolved and corrected 3D delta B0 mapping and fat-water imaging at 7 Tesla. Proc. 29th Annu. Meet. ISMRM, virtual: 2021, p. 1381.
7) Hock, M., Terekhov, M., Stefanescu, M.R., et al. (2020), B0 shimming of the human heart at 7T. Magn Reson Med., 85: 182– 196. https://doi.org/10.1002/mrm.28423
8) Gras, V., Vignaud, A., Amadon, A., Le Bihan, D. and Boulant, N. (2017), Universal pulses: A new concept for calibration‐free parallel transmission. Magn. Reson. Med., 77: 635-643. doi:10.1002/mrm.26148
9) Smith, D.S., Berglund, J., Kullberg, J., Ahlström, H., Avison, M.J., Welch, E.B. Optimization of Fat-Water Separation Algorithm Selection and Options Using Image-Based Metrics with Validation by ISMRM Fat-Water Challenge Datasets. Proc. 21st Annu. Meet. ISMRM, Salt Lake City, Utah: 2013, p. 2413.
10) Prieto, C., Uribe, S., Razavi, R., Atkinson, D. and Schaeffter, T. (2010), 3D undersampled golden‐radial phase encoding for DCE‐MRA using inherently regularized iterative SENSE. Magn. Reson. Med., 64, pp. 514-526, doi:10.1002/mrm.22446
11) Cao, Z., Yan, X. and Grissom, W.A. (2016), Array‐compressed parallel transmit pulse design. Magn. Reson. Med., 76, pp. 1158-1169., doi:10.1002/mrm.26020
Figures