0445
In-vivo Absolute Multinuclear Thermometry (AMT) in a Rat Model1Neuroscience, New York University School of Medicine, New York, NY, United States, 2Bruker cooperation, Billerica, MA, United States, 3Department of Radiology, New York University School of Medicine, New York, NY, United States, 4Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States
Synopsis
We introduce an in-vivo multinuclear MR thermometry technique which utilizes the unique frequency shift between water and sodium resonant frequencies. Reconstruction of the absolute temperature is validated using chemical shift imaging conducted on a rodent model implanted with a thin fiber optic probe for monitoring.
Introduction
Magnetic resonance thermometry (MRT) using the Proton Resonance Frequency (PRF) shift method allows for non-invasive characterization of thermal changes[1]. While MRT using the PRF method is commonly used to track interventions where temperature change is high, measurement of temperature change is greatly challenged by B0 field drift caused by hardware instabilities (e.g., shim and gradient coil heating) and motion artifacts[2]. Measurement of absolute temperature is possible by measuring the spectral distance between the water peak and metabolites such as the commonly used neuronal N-acetylaspartate (NAA)[2]; however, the inherit low concentration of such metabolites (~10mM), signal contamination and other factors hinder using such techniques routinely. Recently, absolute thermometry was introduced in-situ, where the resonance frequency of sodium is used as a reference signal to the resonance frequency of water[3]. The utilization of sodium as a reference frequency rather than proton metabolites is particularly attractive due to larger abundance in many tissues (15-350 nM)[4], short T1 allowing significant averaging, and low spectral contamination[3]. Here, for the first time, we demonstrate in-vivo absolute multinuclear thermometry (AMT) in a live rodent model with an implantable fiber optic temperature probe used to validate our thermometry reconstruction in the brain.Methods
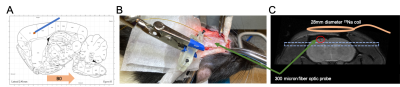
Gold standard temperature measurement & Implantation: temperature validation was conducted using an MR compatible fiber optic probe implanted into the rodent brain (Fig 1A). Measurements were acquired using an FTX-300-LUX+ system (OSENSA Innovations Corp. Burnaby, BC, Canada) equipped with a PRB-100-01M-STM temperature probe having a diameter of 300 micron. This small diameter was used in order to minimize traumatic damage to the brain tissue during surgery that can impact imaging. Surgery was conducted using stereotactic guidance where the probe was implanted at a 30-degree angle relative to the magnetic field to reduce susceptibility artifacts (Fig 1A and B ).Rodent Cooling and heating: spatial and temporally stable temperature was necessary to validate AMT in-vivo. An MR compatible rodent heating pad was connected to a pump outside the scanner room that was placed inside an ice bath. Cold water was used to reduce the temperature of the rodent and three periods of cooling were used. At each step, once the target temperature was reached, the pump was stopped and the brain temperature was allowed to stabilize. After three periods of cooldown a heating period was induced by introduction of warm water through the system. This allowed a random temperature to be reached. At each temperature, shimming was conducted to correct for temperature related susceptibility changes followed by proton and sodium CSI acquisitions.
MR acquisitions and reconstruction: quantification of the resonance frequencies of sodium and proton nuclei was conducted using the chemical shift imaging (CSI) sequence on our institution’s 7T Bruker scanner (Bruker cooperation, Billerica, MA). A custom-built 28-mm in diameter sodium surface coil was used for the sodium acquisitions. The surface coil was placed on the rodent head, which was fixed using ear bars and placed inside an 86mm proton body transmit-receive coil. Before each CSI acquisition a PRESS sequence[5] was used to shim the volume to account for temperature-related susceptibility changes that can change the linewidths. 2D Sodium CSI was acquired with the following parameters: TR=32.73ms, TE=0.673ms, flip angle=60 degrees, spectral width=3200Hz, averages=200, frequency points=100, matrix size 8x8, resolution= 4x4x2.23 mm3, and scan time=418s. Proton CSI was acquired with the following parameters: TR=728.9ms, TE=0.876ms, flip angle=60 degrees, spectral width=1500Hz, averages=3, frequency points=1024, matrix size 8x8, resolution= 4x4x2.23 mm3, and scan time=139s. Upon completion of the acquisitions the data was exported to MATLAB where the frequency difference between the sodium and proton nuclei was computed. Three points in the cooling steps were used to calibrate the model according to reference [3] and the heating step was used to compare the predicted absolute temperature to that measured from the implanted fiber optic probe.
Results
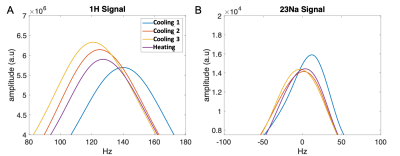
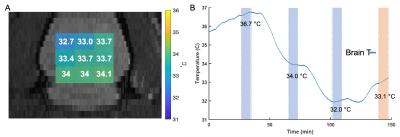
Validation of AMT was conducted using three calibration steps (cooling) and a single random brain temperature attained using a heating step. At each step the B0 shimming was conducted. Sodium and proton spectra at different cooling and heating steps are shown for a single voxel in the brain (Fig 2). While sodium and water signals alone cannot be used to reconstruct absolute temperature, the frequency difference between water and sodium introduces a bijective map onto it. Temperature reconstruction results for nine voxels inside the brain region are shown in fig. 3A. demonstrating a temperature error of 0.5 degrees C compared to the temperature probe with a spatial standard deviation of 0.48 degrees C.Discussions/Conclusions
Here, a first of its kind in-vivo validation of AMT in the brain is conducted in a rodent model. MR temperature measurement using proton and sodium CSI acquisitions were in close agreement with the fiber optic probe measurements. The relatively homogeneous heating pattern is congruent with systemic cooling/heating induced by whole-body cooling or heating.Acknowledgements
We acknowledge support from NIH grant P41 EB0171813.References
1. Ishihara, Y., Calderon, A., Watanabe, H., Okamoto, K., Suzuki, Y., & Kuroda, K. (1995). A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med, 34(6), 814–823.
2. Rieke, V., & Butts Pauly, K. (2008). MR thermometry. J Magn Reson Imaging, 27(2), 376–390.
3. Silletta, E. V, Jerschow, A., Madelin, G., & Alon, L. (2019). Multinuclear absolute magnetic resonance thermometry. Communications Physics, 2(1), 1–10.
4. Madelin, G., & Regatte, R. R. (2013). Biomedical applications of sodium MRI in vivo. Journal of Magnetic Resonance Imaging: JMRI, 38(3), 511–529.
5. Klose, U. (2008). Measurement sequences for single voxel proton MR spectroscopy. European Journal of Radiology, 67, 194–201.
Figures