0444
Correlation between pathophysiological functions of Glioma invasion and water dynamics by FFC-NMR in vivo1University of Torino, Torino, Italy, 2BrainTech Lab INSERM U1205, Grenoble, France, 3CHU Grenoble, Grenoble, France, 4University of Aberdeen, Aberdeen, United Kingdom
Synopsis
R1-dispersion profiles of in vivo glioma mouse models acquired by Fast-Field-Cycling NMR (FFC-NMR) were found to discriminate invasion from proliferation at fields below 2mT. These differences were correlated to the transcytolemmal water-exchange, demonstrating the water cell influx/outflux role in relaxation mechanisms. Hypoxia and H2O2, two major pathophysiological processes of invasion, were demonstrated to modulate relaxation. Immunohistochemistry of aquaporins AQP1 and AQP4 showed that the water-channel proteins were overexpressed in invasion but not in proliferation, suggesting that relaxations at low-field are modulated by water-exchange under the AQP1 and AQP4 control. The method can be extended to FFC imaging.
Introduction
It has been shown that at very low magnetic field (<2mT), R1-dispersion curves of glioma tissues ex vivo exhibit lower relaxation-rate in invasion/migration (Glio6 and the Glio96 mouse models) compared to proliferation (U87 model). These data agree with those observed in vivo in a mouse breast-cancer model1, in which relaxation differences between cancer aggressiveness were attributed to cell transcytoemmal water-exchange changes. Here, we aim to confirm these finding in vivo on glioma models and to establish a link between water dynamic properties, which govern relaxations, and the main pathophysiological processes of glioma invasion. Two FFC-NMR experiments were performed in vivo. First to acquire R1-dispersion profiles that quantify molecular dynamics of water. Second, to measure and compare the intracellular water lifetime parameter (τin). In addition, knowing that hypoxia2 and H2O2 signaling pathway3 play major roles in glioma invasion, functional experiments on U87 cell pellets were performed in vitro to compare R1-dispersion curves and the transmembrane water-exchange of cells under hypoxia and H2O2 stimuli to their controls. Immunohistochemistry (IHC) of AQP4 and AQP1, two water-channel proteins that facilitate the transport of the free water, were analyzed. Our aim is to correlate AQP4 and AQP1 to glioma pathophysiology and more interestingly to relaxation that reflect water dynamics.Subjects and Methods
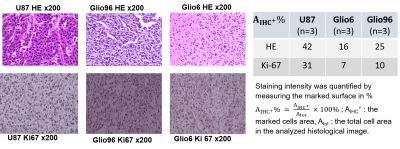
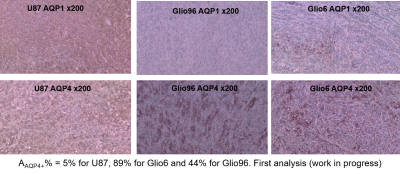
Cells: Glio6 and Glio96 cell were grown under hypoxia 3%O2 while U87 glioma cells under 20%O2. Cell pellets were obtained after centrifugations. Animal models: All procedures were conducted under the (C3818510003) license. In immune-deficient nude-mice (≈35g), 106 human glioma cells (U87, Glio96, Glio6) were injected in the muscle of the hind-limb. In vivo MRI follow-up was performed at 1T, (Aspect M2 Israel) using T2-weighted sequence (Fast Spin Echo sequence, TR/TE=3000/50ms).HE and IHC: HE histology and IHC of Ki-67 were used to assess cellularity and proliferation respectively as well as the IHC of AQP1 and AQP4. These two water-channel proteins were specifically studied because they have been described major actors in glioma invasion and we suppose that they should control transmembrane water-exchange in invasion process. All histological procedures were those used as standard protocols at Grenoble Hospital.
FFC-NMR and Water-exchange in vivo measurements were performed between 0.01 and 16 MHz on Stelar SPINMASTER FFC-NMR relaxometer (40mm 0.5T; Stelar, Mede, Italy) equipped with a dedicated 11mm solenoid RF coil. Saturation recovery method with 16 evolution times were used to describe magnetization recovery. For water-exchange, T1 values at (0.01, 0.02, 0.037, 0.07, 0.15, 0.39, 1 and 10 MHz) were measured with 32 evolution times to improve fast and slow sampling of T1 relaxation recovery.
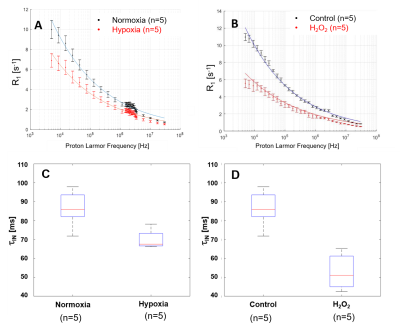
In vitro FFC measurements were realized between 5kHz and 30MHz using a Stelar SPINMASTER-2000 relaxometer with a 10mm bore diameter. Each magnetization was measured over 12 evolution times. Water exchange was measured at a fixed field of 0.5 T with an inversion-recovery sequence of 48 evolutions times on glioma cells in which 9.02 mmol/kg of the paramagnetic Gd-DOTA contrast-agent was added. The experimental procedure necessary for 2SX modelling4 was the same as in reference. R1-dispersion curves were obtained with U87 cells under 20 and 3%O2 and under 5μMol of H2O2. τin of cell pellets were obtained in 2 different experiments: under hypoxia 1%O2 and under H2O2-stimuli (1 to 5 μM) versus without. All data were acquired at 37°C.
Data analysis: R1-dispersion curves were fitted with power-Law5 and the Lorentzian models6 and water-exchange data were analyzed using two-site exchange (2SX) model. Comparison between the different groups was assessed using the Student-test.
Result
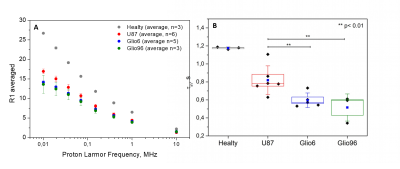
In Fig.1, HE histology and Ki-67-IHC shows a significantly high cellularity and proliferation for U87 than for Glio6 and Glio96, demonstrating phenotypes of proliferation and of invasion respectively. The R1-dispersion curves reported in (Fig.2A) show a lower R1 in Gio6/Glio96 compared with U87. This observation was quantified by model parameters (data not shown here) and corresponded to a decrease of the intracellular water lifetime (τin) (Fig.2B). Fig.3A-B compares the R1-dispersion profiles of U87 cells cultured under hypoxia (3%O2) versus normoxia (20%O2) and under H2O2 stimuli (5µM) and its control respectively. Differences are clearly visible at very low field, demonstrating a relaxation sensitivity to hypoxia and to H2O2 stress. Also, in Fig.3C-D the sensitivity of the transcytolemmal water-exchange to hypoxia and to H2O2 stimuli is demonstrated. In both cases, the intracellular water lifetimes were found to be shorter, and clearly indicate the evident role of these pathophysiological processes on longitudinal relaxations at low fields. These results especially tell us that the contrast observed between invasion and proliferation at very low field (Fig.2A) is very likely linked to hypoxia and H2O2 signaling pathway. AQP4 and AQP1 IHC, which are actors in invasive glioma7, show a strong positivity for Glio6 and Glio96 compared to U87 (Fig.4), suggesting that relaxations at low field are modulated by water-exchange under the control of the AQP1 and AQP4 functions.conclusion
At very low field, water-exchange is demonstrated pivotal in relaxation and relates the mechanisms of relaxation to AQP4 and AQP1 in invasion/migration processes. Our results state that relaxation at very low field as well as the quantitative parameter τin are relevant biomarkers of glioma invasion. The method can be straightforwardly extended to FFC imaging as a new cancer invasion imaging in clinics.Acknowledgements
No acknowledgement found.References
1. Ruggiero MR, Baroni S, Pezzana S, Ferrante G, Geninatti Crich S, Aime S. Evidence for the Role of Intracellular Water Lifetime as a Tumour Biomarker Obtained by In Vivo Field-Cycling Relaxometry. Angew Chemie. 2018;57(25):7468-7472.
2. Monteiro A, Hill R, Pilkington G, Madureira P. The Role of Hypoxia in Glioblastoma Invasion. Cells. 2017;6(4):45.
3. Zhou Y, Wang L, Wang C, Wu Y, Chen D, Lee T. Potential implications of hydrogen peroxide in the pathogenesis and therapeutic strategies of gliomas. Arch Pharm Res. 2020;43(2):187-203.
4. Li X, Mangia S, Lee JH, Bai R, Springer CS. NMR shutter-speed elucidates apparent population inversion of 1H2O signals due to active transmembrane water cycling. Magn Reson Med. 2019;82(1):411-424.
5. Bottomley PA, Hardy CJ, Argersinger RE, Allen moore G. A review of 1H nuclear magnetic resonance relaxation in pathology: Are T1 and T2 diagnostic? Med Phys. 1987;14(1):1-37.
6. Persson B, Malmgren L, Salford L. Studies of 1H-NMR Relaxation Dispersion in Human Brain- tissue Samples: Implications for Magnetic Resonance Relaxation Dispersion Imaging (MARDI). Acta Sci Lund. 2013;2012(001):1-22.
7. Ding T, Ma Y, Li W, et al. Role of aquaporin-4 in the regulation of migration and invasion of human glioma cells. Int J Oncol. 2011;38(6):1521-1531.
Figures