0443
Gauging tumour pressure and vasculature organization via Magnetic Resonance Elastography to grade brain tumours.1LVTS - U1148, University Paris, Paris, France, 2School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3Department of Diagnostic Physics, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 4Department of Biomedical Engineering, King's College London, London, United Kingdom, 5U1021 INSERM, Institut Curie, Paris, France, 6Department of Radiology Ullevål, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 7Department of Neurosurgery, Division of Clinical Neuroscience, Oslo University Hospital, Oslo, Norway, 8Department of Biomedical Engineering and Cardiac Surgery, University of Michigan, Ann Arbor, MI, United States, 9Department of Neuroradiology, Heidelberg University Hospital, Heidelberg, Germany
Synopsis
Distinctive traits of malignant tumours are abnormal angiogenesis and high pressure. Conventional magnetic resonance imaging (MRI) plays a critical role in radiological evaluation of patients and tumour grading, but challenges remain. Pressure and vasculature have a strong impact on the tissue rheology and therefore they can be quantified by Magnetic Resonance Elastography (MRE). We show that MRE allows to quantify non-invasively tumour grade using pressure and tumour vasculature through wave scattering. We believe that MRE could play a central role in tumour grading and diagnosis as well as in therapy planning and dosage, especially in multidrug treatments scenarios.
Introduction
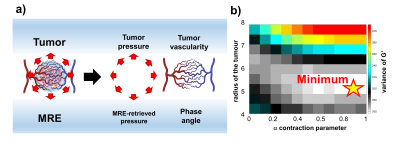
It is well understood that distinctive traits of malignant tumours are abnormal angiogenesis [1], and high pressure [2-4]. These conditions impair perfusion hence anticancer drugs efficacy [5-7] and lead to treatment resistance and enhanced aggressiveness [8, 9]. Characterizing the tumour environment would be crucial for patient management and for the evaluation of treatment effect [10]. Conventional MRI techniques play a critical role in tumours diagnosis. However, challenges remain in the radiological evaluation of patients and tumour grading, and current markers show contradictive results [3,10].Currently, there is no method available to quantify tumour pressure non-invasively [11], and although various methods attempted a quantification of the vasculature organisation, none of them is standardized clinically [12, 13]. In this work we used MRE and tissue biomechanics to characterize tumour pressure and vasculature of a cohort of 26 patients with brain tumours - 13 meningiomas, 13 gliomas (3 grade III and 10 grade IV). We show how one MRE exam provides a comprehensive characterization of the tumour, providing quantification of vascularization and tumour grading non-invasively (Figure 1a).Methods
Patients underwent MRI on a 3T scanner (Signa Premier, GE Healthcare, Waukesha, WI) using a 48-channel head coil. A gravitational transducer [14] placed underneath the subject’s head was used to induce shear waves at 50 Hz into the brain. The MRE acquisition was performed with a multishot gradient-echo MRE sequence using Hadamard encoding [15]. Data inversion [16] allowed to calculate the complex shear modulus G*=G’ +iG’’, where G’ is the shear stiffness, G’’ is the shear viscosity, and Y=2/π*atan(Gl/Gd) in [0,1] is the phase angle. Previously, we demonstrated that scattering is the main mechanism for wave attenuation. Therefore Y offers a unique way to indirectly gauge local vasculature organization (Fig.1a) [9, 10]. Furthermore, the retrieved tissue biomechanics was used to compute the tumour pressure according to [19]. This method was extended to be less sensitive to tumour boundary segmentation errors, introducing an exhaustive parameter search through different tumour radii for finding a global minimum in the search for the tumour pressure (Figure 1b).Results and discussion
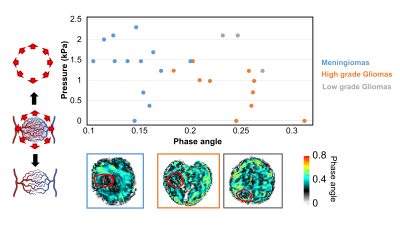
Figure 2 (top) shows for the 26 patients the correlation between tumours’ phase angle with their pressure. Firstly, the phase angle allows to separate meningiomas from gliomas (Figure 2 bottom). This observation is likely to be rooted in the origin of the wave attenuation in MRE which is mainly scattering [17, 18].It is known that gliomas exhibit a substantially different vascular organization than meningioma, which could explain this difference in phase angle [19, 20].Secondly, we observe that high grade gliomas exhibit lower pressures than low grade gliomas. This measurement matches clinical experience: an important feature reflecting the grade of gliomas is their ability to infiltrate the brain parenchyma; this process starts within the vascular network between the white matter tracts and subsequently spreads along the commissural fibres. On the contrary, more confined tumours as meningiomas exhibit elevated pressure, as they commonly push away the brain parenchyma. . In general, the evolution towards a more infiltrative phenotype is mirrored by a reduced amount of pressure exerted on the surrounding, defining tumor pressure as a suitable way of grading tumours.
Conclusions
The characterization of the tumour microenvironment is crucial for tumour characterization and subsequent therapy planning. In this abstract we show that through biomechanics one can assess non-invasively tumour grade using pressure – high grade vs low grade gliomas - and tumour vasculature through wave scattering i.e. phase angle – meningiomas vs gliomas. We believe that MRE could play a central role in tumour grading and diagnosis as well as in therapy planning and dosage, especially in multidrug treatments scenarios.Acknowledgements
No acknowledgement found.References
1. Zarrin, B., et al., Acquired tumor resistance to antiangiogenic therapy: Mechanisms at a glance. J Res Med Sci, 2017. 22: p. 117.
2. Heldin, C.H., et al., High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer, 2004. 4(10): p. 806-13.
3. Gilkes, D.M., G.L. Semenza, and D. Wirtz, Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer, 2014. 14(6): p. 430-9.
4. Kumar, S. and V.M. Weaver, Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev, 2009. 28(1-2): p. 113-27.
5. Jain, R.K., J.D. Martin, and T. Stylianopoulos, The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng, 2014. 16: p. 321-46.
6. Padera, T.P., et al., Pathology: cancer cells compress intratumour vessels. Nature, 2004. 427(6976): p. 695.
7. Bockelmann, L.C. and U. Schumacher, Targeting tumor interstitial fluid pressure: will it yield novel successful therapies for solid tumors? Expert Opin Ther Targets, 2019. 23(12): p. 1005-1014.
8. Jain, R.K., Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell, 2014. 26(5): p. 605-22.
9. Wilson, W.R. and M.P. Hay, Targeting hypoxia in cancer therapy. Nat Rev Cancer, 2011. 11(6): p. 393-410.
10. Liang, J., et al., Monitoring tumour microenvironment changes during anti-angiogenesis therapy using functional MRI. Angiogenesis, 2019. 22(3): p. 457-470.
11. Fovargue, D., et al., Towards noninvasive estimation of tumour pressure by utilising MR elastography and nonlinear biomechanical models: a simulation and phantom study. Sci Rep, 2020. 10(1): p. 5588.
12. Zhang, J., et al., Clinical applications of contrast-enhanced perfusion MRI techniques in gliomas: recent advances and current challenges. Contrast media & molecular imaging, 2017. 2017.
13. Ellingson, B.M., et al., Pseudoprogression, radionecrosis, inflammation or true tumor progression? Challenges associated with glioblastoma response assessment in an evolving therapeutic landscape. Journal of neuro-oncology, 2017. 134(3): p. 495-504.
14. Runge, J.H., et al., A novel magnetic resonance elastography transducer concept based on a rotational eccentric mass: preliminary experiences with the gravitational transducer. Physics in Medicine & Biology, 2019. 64(4): p. 045007.
15. Svensson, S.F., et al., Robustness of MR Elastography in the Healthy Brain: Repeatability, Reliability, and Effect of Different Reconstruction Methods. Journal of Magnetic Resonance Imaging, 2021. 53(5): p. 1510-1521.
16. Sinkus, R., et al., Rheological determinants for simultaneous staging of hepatic fibrosis and inflammation in patients with chronic liver disease. NMR Biomed, 2018. 31(10): p. e3956.
17. Annio, G., et al. About the origin of viscosity in MR-Elastography: tissue absorption or vascular scattering? in ISMRM&SMRT 2021. 2021
18. Sinkus, R., et al., On the origin of frequency power-law for tissue mechanics in elastography. The Journal of the Acoustical Society of America, 2020. 148(4): p. 2601-2601.
19. Schlageter, K.E., et al., Microvessel organization and structure in experimental brain tumors: microvessel populations with distinctive structural and functional properties. Microvascular research, 1999. 58(3): p. 312-328.
20. Assimakopoulou, M., et al., Microvessel density in brain tumors. Anticancer research, 1997. 17(6D): p. 4747-4753.
Figures