0440
MR Elastography of the Frontal Cortex: Impact of Aging and Regional Differences1Mechanical Engineering, University of Delaware, Newark, DE, United States, 2Biomedical Engineering, University of Delaware, Newark, DE, United States, 3Psychology, University of Bath, Bath, United Kingdom
Synopsis
This study tested the utility of high-resolution magnetic resonance elastography (MRE) to analyze frontal cortex degradation due to advancing age. Brain tissue stiffness significantly varied in frontal cortex regions and frontal cortex stiffness also significantly decreased with age. A significant interaction between age and region was found, indicating regions changed differently with age. The stiffness of all frontal cortex regions degraded more per year than reported whole frontal lobe stiffness loss, indicating greater loss in the cortex with age. This approach brings potentially increased sensitivity and specificity to the structure-function relationship found in the frontal cortex with age.
Introduction
The impact of aging on the brain is observed both physically and cognitively. Physical signs of aging include degradation of brain tissue, which notably results in reduced tissue stiffness1. Aging can also contribute to a decline in cognitive function, including executive functions supported by the frontal cortex2. Executive function covers a diverse set of behaviors, including voluntary motor control, working memory, speech production, decision making, and attention modulating2. Assessing neural tissue integrity in aging can help elucidate the mechanisms of cognitive decline and monitor response to cognitive rehabilitation. Magnetic resonance elastography (MRE) has been used to non-invasively study changes in the tissue mechanical properties due to its high sensitivity for assessing microstructural tissue health1. Mechanical properties of brain tissue have been linked to both memory and intelligence performance, indicating so-called viscoelastic structure-function relationships1. Previous MRE research has also shown how stiffness of the frontal lobe decreases with age3. Here we build on that work using high-resolution MRE to examine how aging affects individual regions of the frontal cortex, and specifically consider if regions associated with different cognitive functions decline at different rates.Methods
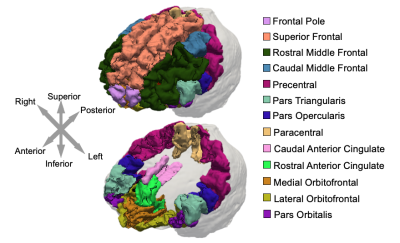
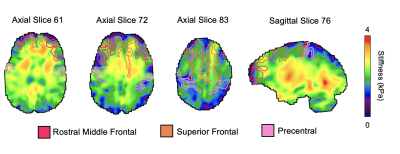
39 healthy older adults aged 60 to 85 (68 ± 5.4 years, 13 males / 26 females) completed the MRI session on a Siemens 3T Prisma, with imaging and analysis protocol optimized for cortical property recovery 4. MRE data acquisition used a 3D multiband, multishot spiral imaging sequence with a 1.25 mm isotropic resolution (TR/TE = 3360/70 ms, 240 × 240 mm2 field-of-view; 192×192 matrix)5. 50 Hz vibrations were applied using the Resoundant pneumatic actuator with pillow driver. The total acquisition time was 10 minutes and 45 seconds. A T1-weighted MPRAGE was used for segmentation of frontal cortical regions. Regions were segmented in Freesurfer, converted to binary masks, and subsequently registered in MRE space using FSL FLIRT6. Regions of interest were the Superior Frontal, Rostral Middle Frontal, Caudal Middle Frontal, Pars Opercularis, Pars Triangularis, Pars Orbitalis, Lateral Orbitofrontal, Medial Orbitofrontal, Precentral, Paracentral, Frontal Pole, Rostral Anterior Cingulate, and Caudal Anterior Cingulate (Figure 1). A nonlinear inversion algorithm (NLI) was used to estimate the complex shear modulus (G = G’ + iG”)7. Storage (G’) and loss moduli (G”) were used to calculate shear stiffness, μ=2|G|2/(G’+|G|). Octahedral shear strain signal-to-noise ratio (OSS-SNR) was also calculated for each participant to confirm that a reliable inversion would be achieved, with a minimum OSS-SNR threshold of 3.08. Representative maps of shear stiffness with regions of interest masks are shown in Figure 2. A linear mixed model with repeated measures, controlling for sex, was used to determine: 1) if shear stiffness differed by region, 2) if there was a significant change in stiffness with age, and 3) if the age relationship differed by region (significant age x region interaction). All statistical analyses were performed in SPSS v1.7.Results
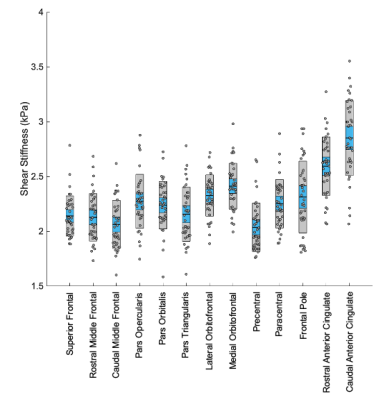
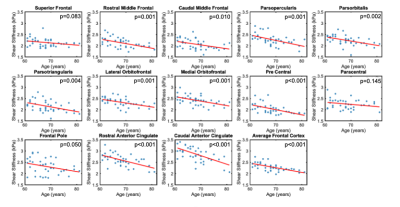
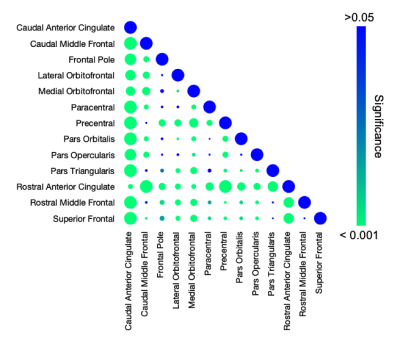
Significant regional differences (p<0.001) in shear stiffness were found with average stiffness of each region ranging between 2.06 kPa (Precentral Gyrus) to 2.85 kPa (Caudal Anterior Cingulate), as shown in Figure 3, which generally agree with previous reports though are softer in this study on older adults9. Stiffness decreased significantly with age (p<0.001) as shown in Figure 4, with an average decrease across all frontal cortex regions was 0.021 kPa/year. The stiffness-age relationship significantly differed between regions (p=0.003), with regional effects ranging from 0.0096 kPa/yr (Superior Frontal Gyrus) to 0.031 kPa/yr (Caudal Anterior Cingulate). Figure 5 presents the differences in average stiffness between regions along with correlation of stiffness between regions across the population.Discussion and Conclusion
The overall decrease in shear stiffness with age in the frontal cortex regions is consistent with established decreases in stiffness across the brain10,11. Annual loss in the average frontal cortex was greater than a previous report considering the entire frontal lobe in a similar older adult population (-0.012 kPa/yr vs. -0.021 kPa/yr in this study)3, indicating greater aging impacts in the cortex than the whole lobe. Decreases found in the frontal regions were also larger than the those found in the hippocampus11. Though both areas contribute to memory performance, the difference in change between the two suggests larger exterior structural changes due to aging than interior structural changes11. The significant interaction effect between region and amount of stiffness loss with age verifies the utility of regional application of MRE to potentially understand the structure-function relationship in the frontal cortex. This approach can add specificity and sensitivity to how mechanical property measures change with age and support cognitive functions. This approach also could provide the sensitivity needed in identifying early onset of neurodegenerative diseases that impact the frontal cortex, such as frontotemporal dementia and Alzheimer’s disease12,13.Acknowledgements
This work was funded by the NIH grant R01-AG058853.References
1. Hiscox, L. V., Schwarb, H., McGarry, M. D. J. & Johnson, C. L. Aging brain mechanics: Progress and promise of magnetic resonance elastography. Neuroimage 232, 117889 (2021).
2. El-Baba, R. M. & Schury, M. P. Neuroanatomy, Frontal Cortex. StatPearls (2021).
3. Arani, A. et al. Measuring the effects of aging and sex on regional brain stiffness with MR elastography in healthy older adults. Neuroimage 111, 59–64 (2015).
4. Hiscox, L. V, McGarry, M. D. J. & Johnson, C. L. Mechanical Property Recovery in the Cerebral Cortex using Magnetic Resonance Elastography. in Proc. Intl. Soc. Mag. Res. Med. (2020).
5. Johnson, C. L., Holtrop, J. L., Anderson, A. T. & Sutton, B. P. Brain MR elastography with multiband excitation and nonlinear motion-induced phase error correction. in Proc. Intl. Soc. Mag. Res. Med. (2016).
6. Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W. & Smith, S. M. FSL. Neuroimage 62, 782–790 (2012).
7. Houten, E. E. W. Van, Miga, M. I., Weaver, J. B., Kennedy, F. E. & Paulsen, K. D. Three-dimensional subzone-based reconstruction algorithm for MR elastography. Magn. Reson. Med. 45, 827–837 (2001).
8. McGarry, M. D. J. et al. An octahedral shear strain-based measure of SNR for 3D MR elastography. Phys. Med. Biol. 56, N153 (2011).
9. Hiscox, L. V. et al. Standard-space atlas of the viscoelastic properties of the human brain. Hum. Brain Mapp. 41, 5282–5300 (2020).
10. Lv, H. et al. MR elastography frequency–dependent and independent parameters demonstrate accelerated decrease of brain stiffness in elder subjects. Eur. Radiol. 30, 6614–6623 (2020).
11. Delgorio, P. L. et al. Effect of Aging on the Viscoelastic Properties of Hippocampal Subfields Assessed with High-Resolution MR Elastography. Cereb. Cortex 31, 2799–2811 (2021).
12. Huston, J. et al. Magnetic resonance elastography of frontotemporal dementia. J. Magn. Reson. Imaging 43, 474–478 (2016).
13. Hiscox, L. V et al. Mechanical property alterations across the cerebral cortex due to Alzheimer’s disease. Brain Commun. 2, (2020).
Figures