0428
Deformable Registration of Serial Micrographs to Post-Mortem MRI in the BigMac Dataset Using TIRL1Wellcome Centre for Integrative Neuroimaging, FMRIB, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom
Synopsis
The BigMac dataset combines extensive post-mortem MRI with histology and polarised light imaging (PLI) in a macaque brain. Adding a new extension to our recently developed platform, the Tensor Image Registration Library (TIRL), we address unique challenges associated with this dataset and successfully align 77 2-D polarised light micrographs to high-resolution structural MRI data.
Introduction
The Big Macaque (BigMac) dataset combines ultra-high angular resolution dMRI at various spatial resolutions and b-values up to 10,000 s/mm2 with histology and polarised light imaging (PLI) in a post-mortem macaque brain1. Accurate registration between all modalities is essential to enable state-of-the-art computational analyses on BigMac after its public release. There are several key challenges associated with data registration in BigMac. First and foremost, BigMac does not include a rigid reference (e.g. a stack of photographs captured while sectioning the brain) from which we can compensate for individual tissue slice deformations. Second, difficulties arise when handling large amounts and a broad spectrum of imaging data. Third, the small brain size and high-resolution imaging necessitate extraordinary precision, on the scale of hundreds of micrometres.Our recently developed software, the Tensor Image Registration Library (TIRL), has demonstrated ability to align stand-alone microscopy slices to post-mortem MRI2 without relying on serial tissue sections. This approach can bypass the need for a rigid reference, making it an ideal tool for BigMac. Here, we make modifications to the existing pipeline to align serial PLI slides to T1-weighted structural MRI of the post-mortem macaque brain. This dataset provides an opportunity to investigate the accuracy of TIRL’s novel slice-to-volume approach using real-life data.
Methods
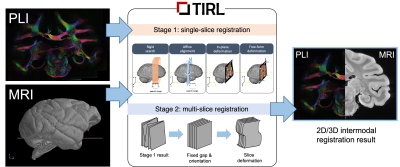
The pipeline (Figure 1) imports the brain-extracted and bias-field corrected T1-weighted 3D ME-GRE volume (voxel size: 0.3x0.3x0.3 mm3) and processed 2D PLI data (the inclination map) at ultrahigh-resolution (4 um/px). The only manual input is required at the start of the first stage. Here the PLI is downsampled to MRI resolution and initialised in a user-defined 10-mm thick rectangular slab, which restricts the search space for subsequent operations that aim to find the best position and orientation of the slice. These operations involve successively more degrees of freedom from rigid, through affine transformations, and finally in-plane and out-of-plane warps. Image cost functions are computed uniformly using the Modality-Independent Neighbourhood Descriptor3 (MIND), to account for the different contrast properties of the inputs. We pass all 77 PLI slices through the first stage, which should provide a reasonably accurate alignment between microscopy and MRI.For BigMac, we extend the above pipeline with Stage 2, which aims to improve the alignment of the individual slices by enforcing them to obey the regularity of the tissue sampling (one slice of PLI at every 350 um). The slices in Stage 2 are treated as an ensemble, whose centre, orientation, and slicing gap are first approximated from the Stage 1 results, then iteratively fine-tuned by a series of bounded local gradient-free optimisations (BOBYQA4), and a gradual, strategic recruitment of the slices.
Finally, we visually compare the accuracy of registrations by Stages 1 and 2, and quantify their difference by taking the mean distance of the 3D locations of corresponding PLI pixels. Previously we showed on simulated data5 that Stage 1 alone could reliably achieve submillimetre accuracy (typically 250 um). Here we gain additional knowledge about the performance of the direct slice-to-volume algorithm by comparing its results against those of a more informed algorithm.
Results and Discussion
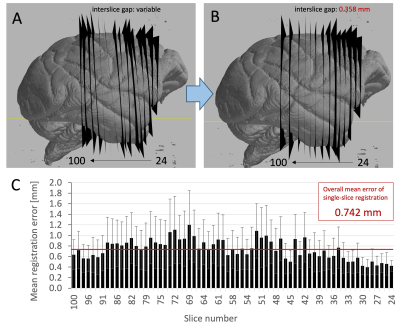
By inspecting the outcomes of Stage 1 and Stage 2 registration, we observed 4 out of 77 cases where Stage 1 failed to provide a reasonable alignment. Stage 2 managed to correct these by having a higher degree of confidence in the starting position and orientation of the four slices, and employing more stringent bounds on their optimisation.In a visual comparison (Figure 2) of 15 representative slices, the observed non-parallel alignments from Stage-1 registrations were sufficiently substantial to create crossovers between immediate neighbours, or leave voxels out, leading to a non-bidirectional mapping between MRI and PLI data. This was successfully addressed by Stage 2.
The “mean registration error” i.e. the pixelwise difference between the output of Stage 1 and Stage 2, indicated a close-to uniform performance on all slices, with an overall accuracy of 0.742 mm. While this may be higher than previously estimated from simulated data (0.25 mm), it shows that the direct slice-to-volume approach is reliable, with comparable performance on real-world imaging data.
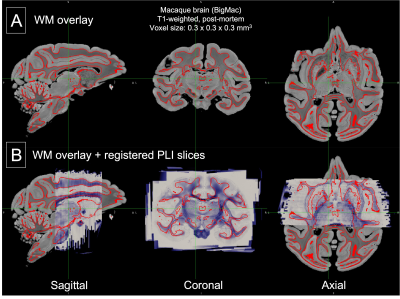
Finally, the reconstruction of 3D PLI data in MR space showed smooth contours along all three orthogonal slicing directions, which were consistent with the boundary of the grey-white matter boundary.
Conclusion
TIRL is a versatile image registration platform that allows submillimetre accurate registration of PLI slices to the post-mortem macaque brain. Future work will focus on applying the revised two-stage algorithm to the full range of microscopy data in the BigMac dataset. This will result in the reconstruction of full 3D microscopy volumes that are inherently aligned with complementary MRI, paving the way for cross-modality and cross-scale analysis of primate brain microstructure.Acknowledgements
This work was supported by the Wellcome Trust (grant WT202788/Z/16/A), EPSRC and MRC (grants EP/L016052/1 and EP/L016052/1). SJ was supported by a Wellcome Senior Research Fellowship (221933/Z/20/Z). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). AH and KM contributed equally to this work.References
[1] Howard A, Jbabdi S, Krapichev AA, Sallet J, Daubney G, Mollink J, Scott C, Sibson N, Miller K. The BigMac dataset: ultra-high angular resolution diffusion imaging and multi-contrast microscopy of a whole macaque brain, 2019, Proceedings of the 27th Annual Meeting of the ISMRM
[2] Huszar IN, Pallebage-Gamarallage M, Foxley S, et al. Tensor Image Registration Library: Automated Non-Linear Registration of Sparsely Sampled Histological Specimens to Post-Mortem MRI of the Whole Human Brain. bioRxiv. 2019.
[3] Heinrich MP, Jenkinson M, Bhushan M, Matin T, Gleeson FV, Brady SM, Schnabel JA. MIND: modality independent neighbourhood descriptor for multi-modal deformable registration. Med Image Anal. 2012 Oct;16(7):1423-35. doi: 10.1016/j.media.2012.05.008. Epub 2012 May 31. PMID: 22722056.
[4] Powell, M.J. (2009). The BOBYQA algorithm for bound constrained optimization without derivatives. Online resource: https://www.damtp.cam.ac.uk/user/na/NA_papers/NA2009_06.pdf, Accessed: 10-Nov-2021
[5] Huszar IN, Miller KL, Jenkinson M. TIRL: Automating Deformable Slice-to-Volume Registration Between Stand-Alone Histology Sections and Post-Mortem MRI, 2021, Proceedings of the 28th Annual Meeting of the ISMRM.
[6] Zhang, Y. and Brady, M. and Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imag, 20(1):45-57, 2001.
Figures