0427
Correction of diffusion pore imaging (DPI) data in the presence of extraporal water and transmembrane water exchange1Deparment of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 2Faculty of Physics and Astronomy, Heidelberg University, Heidelberg, Germany, 3Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 4Molecular Structure Analysis, German Cancer Research Center (DKFZ), Heidelberg, Germany, 5Faculty of Medicine, Heidelberg University, Heidelberg, Germany
Synopsis
Diffusion pore imaging might open a new window into pathologies by providing histology-like information non-invasively by estimating pore size and shape distributions. For current pore imaging approaches, closed pores filled with an NMR visible diffusing medium are assumed, while extraporal components and exchange are neglected, which limits applicability. We propose a method based on Gaussian phase approximation to suppress effects of extraporal fluids and transmembrane water exchange and compare the approach to a filter-based method. Thus, one main obstacle for in vivo applications is reduced, the required high gradient amplitudes may be obtained using local gradient coils in the future.
Introduction
Diffusion pore imaging1 (DPI) so far was mostly conducted in artificial phantom systems that excluded extraporal signal contributions. In last year’s ISMRM abstract2 we were able to show that by adding filter gradients to the original long-narrow scheme, it is indeed possible to acquire diffusion pore images in the presence of extraporal signals. However, this still implies negligible exchange between the intra- and extraporal space, and the resulting gradient scheme is quite sophisticated. Therefore, an easier solution based on the Gaussian phase approximation is proposed in this study that is applicable for the correction of both extraporal signal contributions and effects originating from exchange between intra- and extraporal space.Methods
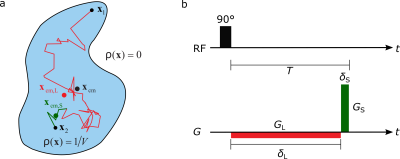
The specialized diffusion encoded sequence used for DPI is depicted in Figure 1a. This approach is called the long-narrow gradient scheme and the refocusing condition for the gradients needs to be fulfilled:
$$\int^{T}_{0}\vec{G}_{\delta_\text{L},\delta_\text{S}}(t)dt=0$$
Considering multiple pores with no extraporal detectable medium, in the limit of $$$\delta_\text{L}\rightarrow\infty,\delta_\text{S}\rightarrow0$$$, the average pore space function (PSF) of the pores illustrated in Figure 1b can be reconstructed from the measured signal $$$S_{\infty,0}(\vec{q})$$$ via a simple inverse Fourier transform:
$$\rho_\text{avg}(\vec{x})=iFT(S_{\infty,0}(\vec{q}))(\vec{x})=\sum^{M}_{n=1}f_n\rho_n(\vec{x}-\vec{x}_\text{CM})$$
where $$$\rho_n(\vec{x}-\vec{x}_{\text{CM}})$$$ is the pore space of the n-th pore shifted to the origin, $$$f_n=V_n/V$$$ is the respective volume fraction within the total intraporal volume $$$V$$$ and $$$\vec{q}=\gamma\vec{G}_\text{L}\delta_\text{L}=\gamma\vec{G}_\text{S}\delta_\text{S}$$$. If also extraporal signals of an NMR-visible medium are allowed and assuming that the extraporal component follows the Gaussian phase approximation3, the signal can be modeled using $$$S_\text{extra}(\vec{q})=S_{0,\text{extra}}e^{-\vec{q}^2c_1}$$$ where $$$c_1$$$ is a constant for radial acquisitions in $$$q$$$-space. The projections in $$$x$$$-space obtained from radial acquisitions can then be written as
$$S(x)=S_0[f_\text{e}e^{-\vec{x}^2c_2}+(1-f_\text{e})iFT(S_{\delta_\text{L},\delta_\text{S}}(\vec{x})]$$
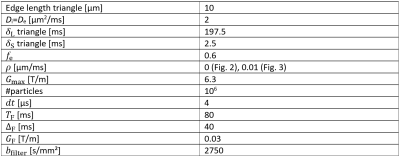
where $$$f_\text{e}=V_\text{e}/V_\text{total}$$$ is the extraporal volume fraction and a constant $$$c_2$$$. If the signal drop of the extraporal component is significantly faster than the intraporal one, in the long-time limit $$$e^{-\vec{x}^2c_2}$$$ becomes broad in $$$x$$$-space and can be fitted by a Gaussian for retrospective removal of the contamination by subtracting it from the actual projection of the PSF. Simulations were carried out using our in-house developed Monte-Carlo simulation tool4 implemented in MATLAB. Simulation parameters used for the equilateral triangles can be found in Table 1. Thirty-two gradient directions were simulated in order to be able to reconstruct the two-dimensional pore space images using an inverse Radon transformation.
Results
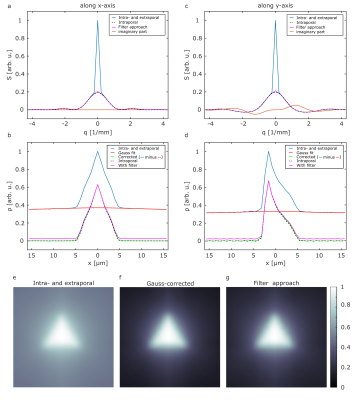
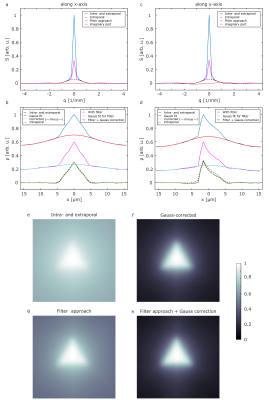
Figure 2 shows the simulated signals (a,c) and corresponding projections of the PSF (b,d) for two representative gradient directions (x-,y-direction). It is obvious that the extraporal signal leads to a deviation for small values that results in a baseline in the reconstructed projections of the PSF and in the pore image without any correction (e). The filter approach (magenta) that we previously proposed is in good agreement with the simulation of only intraporal contributions for both the signal and the reconstructed projection. However, a small remaining baseline is still present. The Gauss-corrected projection of the PSF shows no deviation from the intraporal simulation and yields a slightly higher contrast in the reconstructed image (f) when compared to the filter approach (g). Simulations also considering permeable membranes of the triangles are shown in Figure 3. When comparing the signals in Fig. 3(a,c) to the ones in Figure 2(a,c), it is clearly visible that the deviations from the expected signal are quite severe. Using the filter approach (magenta) the deviation gets smaller; however, a quite significant deviation remains. This deviation carries on to the projections of the PSF (b,d) and the reconstructed images (e,g). When applying the proposed Gauss correction to both the direct measurement and the filter approach, it is indeed possible to obtain the projection of the PSF with almost no deviation from intraporal simulations. This also translates to reconstructed pore images (f,h) that are indistinguishable from the ones presented in Figure 2.Discussion and Conclusion
Using our proposed correction method, the simulations indicate that it is indeed possible to also apply DPI to pores that are not completely closed, thereby simplifying the acquisition of pore imaging data in the presence of extraporal signals and exchange. For very low packing densities, the extraporal contribution to the signal is rather large. Since the extraporal diffusion is very likely to be Gaussian in that case, it can easily be removed by one of our proposed methods. When considering larger packing densities, the extraporal contributions may become very small; nevertheless, these contributions will be harder to remove since the signal in the extraporal space will also decay much slower and the Gaussian phase approximation may not be valid anymore. Regarding exchange, the applicability will be limited by the necessity to reach the long-time limit within the pores. While general limitations such as the high necessary gradient amplitude are still a concern for the application of DPI for in vivo measurements, cell samples or small animals on preclinical systems represent the natural next step to be investigated using the correction methods presented here. Regarding human in vivo applications, especially local gradient coils might open a perspective due to the high gradient amplitudes they may provide, potentially opening a new window for further insight into pathologies5,6.Acknowledgements
No acknowledgement found.References
[1]: Laun, F.B., et al., „Determination of the Defining Boundary in Nuclear Magnetic Resonance Diffusion Experiments”, PRL, 107 (2011)
[2]: Ludwig, D., et al. “Filtered water diffusion pore imaging on a 14.1 T spectrometer using strong gradients and capillary phantoms in the presence of extraporal fluid.”, Proc. Intl. Soc. Mag. Reson. Med. 29: 3416, (2021)
[3]: Grebenkov, D.S., et al., “NMR survey of reflected Brownian motion.”, Rev Mod Phys, 79 (2007) 1077-1137.
[4]: Ludwig, D., et al., “Apparent exchange rate imaging: On its applicability and the connection to the real exchange rate”, MRM, 86.2 (2021): 677-692
[5]: Littin, S., et al., “Approaching order of magnitude increase of gradient strength: Non-linear breast gradient coil for diffusion encoding“, Proc. Intl. Soc. Mag. Reson. Med. 29: 3096, (2021)
[6]: Jia, F., et al., „Design of a high-performance non-linear gradient coil for diffusion weighted MRI of the breast“, JMR, 331, (2021)
Figures