0426
A coil-based approach to accelerate the FLIP spatial lipid removal algorithm in 3D EPSI.1Hoglund Biomedical Imaging Center, University of Kansas Medical Center, Kansas City, KS, United States, 2Department of Neurology, University of Kansas Medical Center, Kansas City, KS, United States, 3Department of Radiology, University of Kansas Medical Center, Kansas City, KS, United States, 4Department of Molecular & Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, United States
Synopsis
We have recently developed the spatial domain, Fast LIpid signal Processing (FLIP) algorithm to remove subcutaneous lipid signals in MRSI. Practical application of FLIP to 3D EPSI was challenging due to the need for long processing times. We present an updated algorithm, named FLIP-COIL, which significantly reduces the processing times utilizing receive coil senstivity profiles. Algorithms including FLIP, FLIP-COIL, as well as PGA, HSVD and L2 are compared in 3D EPSI of ten subjects. This study demostrates that the FLIP-COIL approach can drastically reduce processing time with favorable performance of lipid removal in 3D EPSI over existing other algorithms.
INTRODUCTION
Recent advancements in MRSI have aimed to achieve high resolution and increased spatial coverage encompassing the whole brain, including the cortical regions near the scalp. However, lipid signals originating from the scalp have continued to pose challenges in the acquisition and quantification of metabolite and macromolecule spectra. We have developed the Fast Lipid signal Processing (FLIP) algorithm1 which removes subcutaneous lipid contributions in MRSI data based on scalp and brain structural information from MRI. While this approach is very fast for 2D MRSI, scaling up to high resolution 3D data posed difficulty as the number of independent mask voxels in the model grows very large, requiring long processing time. To reduce the 3D EPSI processing time, we aimed to develop a spatial basis with fewer terms without sacrificing lipid removal capacity. Considering the strong lipid signal attenuation with distance from relatively small receiver elements, the spatial domain could be reduced to the region near each receiver coil element, rather than solving the global whole-head geometry. We compared this approach, dubbed FLIP-COIL, with the FLIP algorithm as well as the Papoulis-Gerchberg (PG) algorithm2, Hankel Singular Value Decomposition (HSVD)3, and L2 lipid removal4.METHODS
All MR measurements were performed on a 3 T scanner (Skyra, Siemens, Erlangen, Germany) using a 16-channel head receive coil. MRSI (3D) data were acquired using an EPSI sequence5 (TE/TR/TR2=16/1551/511 ms, matrix = 50×50×18, FOV = 280×280×180 mm3, 76% GRAPPA fill factor (38/50 lines), no inversion lipid nulling, acquisition time = 17.8 min). Unsuppressed water MRSI data were acquired for coil combination and a concentration reference. T1-weighted MRI was performed using a MPRAGE sequence (TE/TR/TI = 3.98/2000/830 ms, matrix = 176×256×256, FOV = 176×256×256 mm3, GRAPPA factor = 2). Ten subjects (mean age = 33 yrs, F/M=4/6) were scanned, and all were consented in accordance with protocols approved by the University of Kansas Medical Center Institutional Review Board. EPSI data regridding was performed using the MIDAS software.6 All lipid removal algorithms were implemented in-house in Matlab (Mathworks, Inc., MA, USA).The FLIP methodology has been described in our recent work1. For the FLIP-COIL approach, an additional mask preparation process was used. First, the SNR was determined from the water reference MRSI and then interpolated to the MRI dimensions. An SNR threshold was then found for each coil such that 1500 to 2000 lipid voxels remained, which is ~1/10 of FLIP. Processing times were recorded, and spectra for each algorithm were fitted using LCModel to assess N-Acetylaspartate (NAA), total choline (tCho) and creatine (Cr) concentrations. Fitting performance was evaluated by the Cramer Rao Lower Bound (CRLB) for NAA in four supratrentorial transverse slices. For CRLB assessment, voxels were subject to criteria of tissue fraction ≥50%, linewidth ≤ 0.1 ppm and CRLB of NAA ≤ 20% (all methods). Metabolite concentration ratios were assessed in gray matter (GM) and white matter (WM) regions in a superior slice in 10 subjects, subject to the same voxel inclusion criteria.
RESULTS AND DISCUSSION
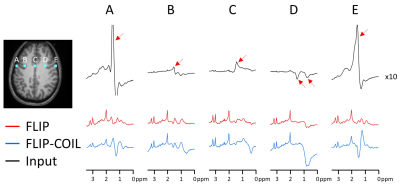
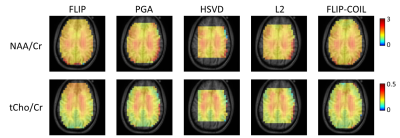
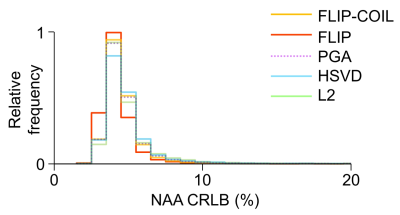
The efficiency of lipid removal and variations in the lineshape quality using FLIP-COIL are shown in Fig. 1. Overall, the FLIP based methods (FLIP, FLIP-COIL) performed better in lipid removal than other methods (Fig. 2). NAA/Cr and tCho/Cr values were similar with expected GM and WM differences using all algorithms, (Fig. 2). HSVD and L2 oucomes showed many missing outer cortical voxels indicating failed spectral fitting due to the poor spectral quality (i.e., residual lipid) (Fig. 2).Spectral quality after lipid removal was evaluated in ten subjects using the Cramer Rao Lower Bound (CRLB) provided by LCModel (Fig. 3). In total, 10,298 voxels passed the quality criteria. The distribution of CRLB showed was most left-shifted, i.e., better spectral quality, with the original FLIP algorithm, while FLIP-COIL showed somewhat worse CRLB but still better than PGA, L2 and HSVD (Fig. 3).
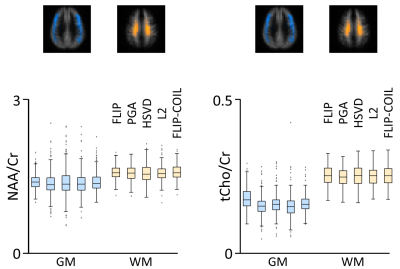
Metabolite concentration ratios of NAA and tCho to Cr were assessed from 343 GM voxels (GM fraction: 67%) and 316 WM voxels (WM fraction: 91%) (Fig. 4). Central WM regions showed higher NAA/Cr and tCho/Cr than those in the outer GM with all algorithms, as expected. Generally, FLIP had the narrowest distributions and fewest outliers with comparable outcomes by FLIP-COIL. The PGA, HSVD and L2 algorithms generally exhibited broader distributions and more outliers (Fig. 4).
Processing times and methodology were previously reported1 for FLIP, PGA, HSVD and L2. The newly developed FLIP-COIL approach improved the processing time from 40 min (for FLIP) to 11 min.
In summary, we have developed a new approach to accelerate processing of the spatial lipid signal removal for 3D EPSI. The coil-wise processing approach of FLIP-COIL out-performed other frequently used algorithms, and was largely comparable with the FLIP benchmark. By restricting the basis to match the expected signal components from each coil and drastically reducing the matrix size, FLIP-COIL could achieve approximately 4 times acceleration of processing speed with minimal impact on lipid removal performance of FLIP.
Acknowledgements
This study is partially supported by NIH (R01 AG060050). The Hoglund Biomedical Imaging Center is supported by the NIH (S10RR029577) and the Hoglund Family Foundation.References
1. Adany P, Choi I-Y, Lee P. Method for fast lipid reconstruction and removal processing in 1H MRSI of the brain. Magn Reson Med. 2021;00:1–15. https://doi.org/10.1002/mrm.28949
2. Plevritis SK, Macovski A. Spectral extrapolation of spatially bounded images. IEEE transactions on medical imaging 1995;14(3):487-497.
3. Barkhuijsen H, Debeer R, Vanormondt D. Improved Algorithm for Noniterative Time-Domain Model-Fitting to Exponentially Damped Magnetic-Resonance Signals. J Magn Reson 1987;73(3):553-557.
4. Bilgic B, Chatnuntawech I, Fan AP, Setsompop K, Cauley SF, Wald LL, Adalsteinsson E. Fast image reconstruction with L2-regularization. Journal of magnetic resonance imaging : JMRI 2014;40(1):181-191. 5. Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, Bloomer C. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med. 2009;61(3):548-559.
6. Maudsley AA, Darkazanli A, Alger JR, Hall LO, Schuff N, Studholme C, Yu Y, Ebel A, Frew A, Goldgof D, Gu Y, Pagare R, Rousseau F, Sivasankaran K, Soher BJ, Weber P, Young K, Zhu X. Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. Nmr Biomed 2006;19(4):492-503.
Figures