0425
ReImagining the Young Adult Human Connectome Project (HCP) Diffusion MRI Dataset1QMI, NIBIB/NIH, Bethesda, MD, United States, 2Natbrainlab, King's College London, London, United Kingdom, 3Laboratory of Neurobiology, Department of Cell and Developmental Biology, University College London, London, United Kingdom
Synopsis
The Human Connectome Project (HCP) has brought significant advancements in hardware, acquisition, and preprocessing. Even after a decade since its collection, the HCP diffusion MRI data is still relevant for its richness and high resolution. Noise and geometric distortions, however, are particularly pronounced in this dataset. In this work, we have reprocessed nearly the entire HCP dMRI dataset while applying several recent processing improvements. We compared the quality of the newly processed dMRI outputs to the release version. We observed clearly detectable improvements. The data originated from this new processing will be made publicly available.
Introduction
Even after a decade since its collection, the young adult Human Connectome Project (HCP)1,2 remains the source of one of the largest high resolution and high quality publicly available diffusion MRI (dMRI) datasets. Key acquisition advancements of this dataset included: strong gradients, multi-band, high resolution, and 2-way reversed phase-encoding. The original dMRI preprocessing included novel techniques such as predictive model for motion&eddy current-induced image distortions3, blip-up blip-down susceptibility distortion correction4, and replacement of signal outliers5,6.The past decade has witnessed several methodological and technical advancements in post-acquisition strategies to improve the quality of diffusion weighted images (DWI). The application of these dMRI preprocessing advancements to the HCP data has the potential to further improve HCP data quality, increasing its validity for addressing biological questions. We have developed a dedicated pipeline, specifically optimized for the HCP dMRI data, that includes steps affecting the correction of: susceptibility-induced distortion, motion effect on gradient nonlinearity, Gibbs ringing, signal drift, as well as denoising of the images.With this pipeline we have reprocessed the entire HCP dMRI dataset and we present here representative results of the processed DWIs, computed dMRI-derived maps, and fiber tractography. Population atlases derived from these images were also produced using a tensor-based registration approach.Materials
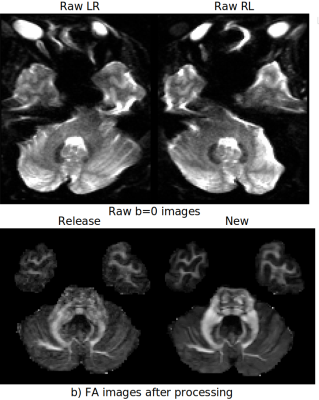
PreProcessing: The processing pipeline is a version of the TORTOISE7,8, specially tailored to HCP. The improvement areas include:- Susceptibility-induced distortions: The unprocessed HCP dataset was acquired with a very high resolution at the expense of severe EPI distortions. In the processed version, residual distortion effects were still present in the data of several subjects. The field of blip-up blip-down EPI distortion correction has seen considerable improvements in the past decade. For this work, we applied the DRBUDDI method9, which was recently shown to be one of the superior distortion correction techniques10 thanks to its ability to take advantage of not only of the b=0s/mm2 images but also of the diffusion tensors, in addition to constraining the correction with an undistorted T2W structural image. The 3D T2W structural image from HCP was unideal for DRBUDDI, therefore, a machine learning-based technique, SynB0-Disco11 was used to generate a suitable constraint image.
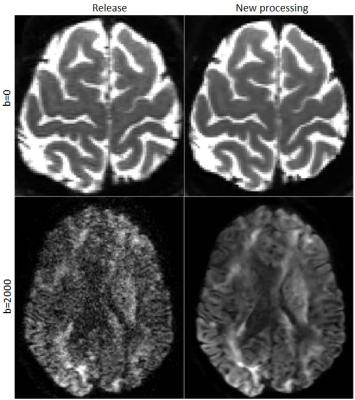
- Denoising: Denoising has been one of the focus areas in dMRI preprocessing in the past decade and several methodologies that aim to remove the noise without blurring or introducing additional bias to the data have been proposed12,13,14,15. In this work, after empirical experimentation, we opted to use the technique proposed by Veraart12 with a kernel radius of 3.
- Gibbs-ringing: Even though HCP data has 6/8 k-space coverage, we observed improvements with the subvoxel-shift method16 without introducing additional imperfections. The current reprocessed data has this method applied, however, the recently developed technique by Lee17 will be the method of choice for the final release.
- Gradient nonlinearity: The released HCP data already provided “gradwarped” DWIs and the voxelwise gradient-deviation tensor images. Using a single gradient-deviation tensor for all DWIs disregards the effects of inter-volume motion. In this work, we also computed the voxelwise Bmatrices, which actually consider such effects18.
- Signal Drift: Signal drift due to the length of the scan was observed in the HCP data, therefore, their effects were removed with a recent method19.
- Output resolution and templates: The processed DWIs were output at both the original 1.25mm isotropic resolution and at 1mm resolution at the space of the processed T1W image. A diffusion tensor-based registration and atlas creation20,21 was performed using the 1mm data and the warped versions of the DWIs on the template space were also generated.
Results
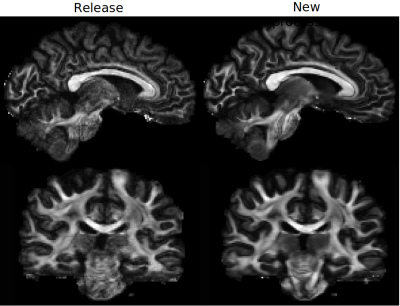
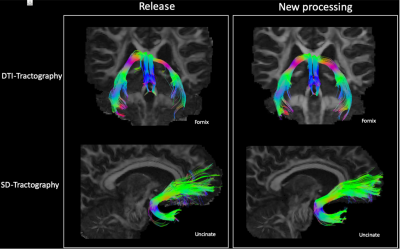
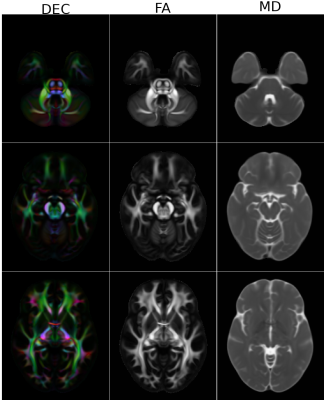
Figure1 displays the effects of denoising on DWIs. Both low and high b-value images have significantly improved contrasts compared to the HCP Release version. Figure2 displays an FA map near the level of the Pons to illustrate the improved susceptibility distortion correction. The anatomy in the brainstem, specifically corticospinal tracts, is more accurate with the new processing. Additionally, the new processing provides more detailed anatomy in the temporal lobes. Figure3 displays the effects of the processing on FA maps of a representative subject, in coronal and sagittal views. Figure4 displays fiber tractography results from the Uncinate (spherical deconvolution) and Fornix (diffusion tensor) from a single subject. Uncinate tracts are more compact and less artifactual while the Fornix tracts have better anatomical symmetry with the new processing. Figure5 displays dMRI-derived scalar maps from the atlas computed using the newly processed data. The atlas is of very high quality with FA and MD values being representative of the average of individual subjects’ values in corresponding regions.Conclusion
In this work, we have reprocessed the HCP1200 dataset by applying novel preprocessing techniques that became available since its release. The newly processed native-space DWIs, template-space DWIs and the templates will be made publicly available through a dedicated site in the future. Users who would like to access the data before its availability in this new site can contact the authors at https://tortoise.nibib.nih.gov.Acknowledgements
This research was supported by the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering and National Institute of Neurological Disorders and Stroke in the National Institutes of Health. The contents of this work do not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred. This work was supported by a Wellcome Trust Investigator Award (No. 103759/Z/14/Z).References
1.Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, Della Penna S, Feinberg D, Glasser MF, Harel N, Heath AC, Larson-Prior L, Marcus D, Michalareas G, Moeller S, Oostenveld R, Petersen SE, Prior F, Schlaggar BL, Smith SM, Snyder AZ, Xu J, Yacoub E; WU-Minn HCP Consortium. The Human Connectome Project: a data acquisition perspective. Neuroimage. 2012 Oct 1;62(4):2222-31. doi: 10.1016/j.neuroimage.2012.02.018.
2. Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, Glasser MF, Hernandez M, Sapiro G, Jenkinson M, Feinberg DA, Yacoub E, Lenglet C, Van Essen DC, Ugurbil K, Behrens TE; WU-Minn HCP Consortium. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage. 2013 Oct 15;80:125-43. doi: 10.1016/j.neuroimage.2013.05.057. Epub 2013 May 20. PMID: 23702418; PMCID: PMC3720790.
3. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016 Jan 15;125:1063-1078. doi: 10.1016/j.neuroimage.2015.10.019. Epub 2015 Oct 20. PMID: 26481672; PMCID: PMC4692656.
4. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003 Oct;20(2):870-88. doi: 10.1016/S1053-8119(03)00336-7. PMID: 14568458.
5. Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage. 2016 Nov 1;141:556-572. doi: 10.1016/j.neuroimage.2016.06.058. Epub 2016 Jul 5. PMID: 27393418.
6. Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, Bastiani M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage. 2017 May 15;152:450-466. doi: 10.1016/j.neuroimage.2017.02.085. Epub 2017 Mar 8. PMID: 28284799; PMCID: PMC5445723.
7. Pierpaoli C, Walker L, Irfanoglu MO, Barnett AS, Chang LC, Koay CG et al. TORTOISE: An integrated software package for processing of diffusion MRI data. In Proceedings of International Society of Magnetic Resonance in Medicine; 2010, p.1597.
8. Irfanoglu MO, Nayak A, Jenkins J, Pierpaoli C. TORTOISEV3: Improvements and New Features of the NIH Diffusion MRI Processing Pipeline, In Proceedings of International Society of Magnetic Resonance in Medicine; 2017, p.3540.
9. Irfanoglu MO, Modi P, Nayak A, Hutchinson EB, Sarlls J, Pierpaoli C. DR-BUDDI (Diffeomorphic Registration for Blip-Up blip-Down Diffusion Imaging) method for correcting echo planar imaging distortions. Neuroimage. 2015 Feb 1;106:284-99. doi: 10.1016/j.neuroimage.2014.11.042. Epub 2014 Nov 26. PMID: 25433212; PMCID: PMC4286283.16.
10. Gu X, Eklund A. Evaluation of Six Phase Encoding Based Susceptibility Distortion Correction Methods for Diffusion MRI. Front Neuroinform. 2019 Dec 5;13:76. doi: 10.3389/fninf.2019.00076. PMID: 31866847; PMCID: PMC6906182.17.
11. Schilling KG, Blaber J, Huo Y, Newton A, Hansen C, Nath V, Shafer AT, Williams O, Resnick SM, Rogers B, Anderson AW, Landman BA. Synthesized b0 for diffusion distortion correction (Synb0-DisCo). Magn Reson Imaging. 2019 Dec;64:62-70. doi: 10.1016/j.mri.2019.05.008. Epub 2019 May 7. PMID: 31075422; PMCID: PMC6834894.
12. Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory, Magnetic Resonance in Medicine, 2016, 76 (5), 1582-1593.
13. Moeller S, Pisharady PK, Ramanna S, Lenglet C, Wu X, Dowdle L, Yacoub E, Uğurbil K, Akçakaya M. NOise reduction with DIstribution Corrected (NORDIC) PCA in dMRI with complex-valued parameter-free locally low-rank processing. Neuroimage. 2021 Feb 1;226:117539. doi: 10.1016/j.neuroimage.2020.117539. Epub 2020 Nov 10. PMID: 33186723; PMCID: PMC7881933.
14. Ma X, Uğurbil K, Wu X. Denoise magnitude diffusion magnetic resonance images via variance-stabilizing transformation and optimal singular-value manipulation. Neuroimage. 2020 Jul 15;215:116852. doi: 10.1016/j.neuroimage.2020.116852. Epub 2020 Apr 17. PMID: 32305566; PMCID: PMC7292796.
15. Fadnavis, J. Batson, E. Garyfallidis, Patch2Self: Denoising Diffusion MRI with Self-supervised Learning, Advances in Neural Information Processing Systems 33 (2020)
16. Kellner E, Dhital B, Kiselev VG, Reisert M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. 2016 Nov;76(5):1574-1581. doi: 10.1002/mrm.26054. Epub 2015 Nov 24. PMID: 26745823.
17. Lee H, Novikov DS, Fieremans. Removal of Partial Fourier-Induced Gibbs (RPG) Ringing artifacts in MRI, In Proceedings of International Society of Magnetic Resonance in Medicine; 2021, p.3537.
18. Vos SB, Tax CM, Luijten PR, Ourselin S, Leemans A, Froeling M. The importance of correcting for signal drift in diffusion MRI. Magn Reson Med. 2017 Jan;77(1):285-299. doi: 10.1002/mrm.26124. Epub 2016 Jan 29. PMID: 26822700.
19. Rudrapatna U, Parker GD, Roberts J, Jones DK. A comparative study of gradient nonlinearity correction strategies for processing diffusion data obtained with ultra-strong gradient MRI scanners. Magn Reson Med. 2021 Feb;85(2):1104-1113. doi: 10.1002/mrm.28464. Epub 2020 Oct 3. PMID: 33009875; PMCID: PMC8103165.
20. Irfanoglu MO, Nayak A, Jenkins J, Hutchinson EB, Sadeghi N, Thomas CP, Pierpaoli C. DR-TAMAS: Diffeomorphic Registration for Tensor Accurate Alignment of Anatomical Structures. Neuroimage. 2016 May 15;132:439-454. doi: 10.1016/j.neuroimage.2016.02.066. Epub 2016 Feb 28. PMID: 26931817; PMCID: PMC4851878.
21. Irfanoglu MO, Nayak A, Pierpaoli C. Diffusion MRI Atlases from the Human Connectome Project Data, , In Proceedings of International Society of Magnetic Resonance in Medicine; 2020, p.3751.
Figures