0424
Laminar Myeloarchitectonic Mapping using T1- and T2-weighted MRI in Macaque Monkeys1Center for Biosystems Dynamics Research, RIKEN, Kobe, Japan, 2Inserm, Stem Cell and Brain Research Institute, Lyon, France, 3State Key Laboratory of Neuroscience, Institute of Neuroscience, Shanghai, China, 4Department of Neuroscience, Washington University School of Medicine, St Louis, MO, United States, 5Department of Neuroscience, Washington University School of Medicine, St Louis, MT, United States
Synopsis
Classically, many cortical areas have been defined by their distinctive myeloarchitectonic laminar profiles. Here, we explore laminar transitions by adjusting the structural MRI resolution according to the average width of standard six cortical layers and by attenuating Gibbs’ ringing artefact using T1w/T2w-FLAIR ratio in anesthetized macaque monkeys. We demonstrate that laminar similarity measures, together with the ‘Human Connectome Project’-style midthickness weighted gradients, reveal sharp transitions between several cortical areas in heavily and lightly myelinated brain areas providing mutually supportive information of cortical organization.
Introduction
Cortical areas have often been defined by their distinctive laminar myeloarchitecture using invasive histology (1,2). More recent studies have determined areal transitions using cortical midthickness weighted T1w/T2w ratio MRI in living primates including humans (3,4,5,6). Thus, MRI studies of areal transitions might also benefit from laminar investigations, however, Gibbs’ ringing which can manifest as cortical layer-like image artefact may complicate such efforts. Here, we present a modified T1w/T2w myelin imaging strategy designed for laminar myeloarchitectonic mapping in macaque monkeys in vivo. More specifically, we demonstrate that by matching T1w and T2w-FLAIR k-space acquisition, the Gibbs’ ringing artefact can be effectively attenuated using a T1w and T2w-FLAIR ratio, thereby yielding improved laminar tissue contrasts while concurrently enhancing the contrast to intra-cortical myelin.Methods
Experiments were performed using a 3T MRI scanner (Prisma, Siemens) equipped with a custom-made 24-channel coil for macaque brain (5). The experiments were conducted in accordance with the institutional guidelines for animal experiments (MA2008-03-11). Macaque monkeys (N=5, total trials=7) were sedated with intramuscular injection of dexmedetomidine and ketamine and anesthesia was maintained using 1.2–1.5 % isoflurane.Image acquisition resolution was scaled according to median cortical thickness divided by six cortical layers (1.9 mm / 6=0.32 mm). T1w images were acquired using 3D MPRAGE (0.32 mm isotropic, averages=12, TI=900 ms, GRAPPA=2) whereas T2w images were acquired using SPACE-FLAIR (0.32 mm isotropic, averages=6, TI=1800 ms, TR= 5 s, GRAPPA=2). The acquisition time for each session was ≈3 hours.
Data preprocessing, including image registration, bias-field correction, distortion correction and cortical surface generation, was performed using the non-human primate version of the Human Connectome Project (NHP-HCP) pipelines (5,6). Twelve equivolume laminar surfaces were generated using the WorkBench command ‘-surface-cortex-layer’ in the native AC-PC space. Each subjects averaged T1w, T2w-FLAIR and T1w/T2w-FLAIR images were spline interpolated to 0.25 mm resolution and then mapped to the native mesh using WorkBench command ‘-volume-to-surface-mapping’ with the ribbon-constrained algorithm. Subjects were registered using the MSMSulc surface algorithm and the data was resampled to a dense 164k atlas mesh in CIFTI format. Contrasts were normalized, concenated across subjects and laminar similarity across the cortical mantle was evaluated using Fisher-z converted Pearson’s correlation.
Results
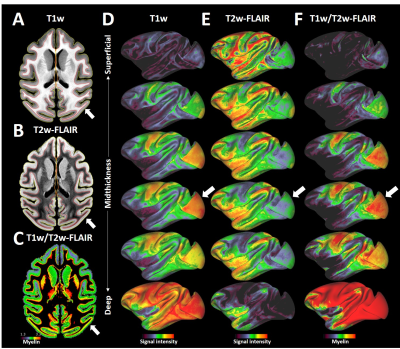
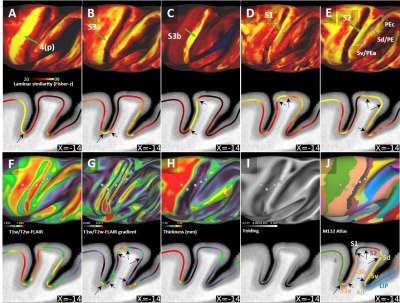
Average T1w cortical surface depth profiles exhibited relatively high signal-intensity (SI) at deep cortical-profiles and progressively lower SI towards pial surface (Fig. 1A, D). In contrast, T2w-FLAIR exhibited low and high signal intensity at deep and superficial layers, respectively (Fig. 1B, E). These results are in line with signal intensities between white matter and grey matter and well-established cortical myeloarchitecture, suggesting that these cortical-depth contrast variations are mainly driven by myelination. The laminar MRI profiles revealed non-monotonic and rich myeloarchitectonic details throughout the cortical mantle. For example, the densely myelinated external band of Baillarger in V1 was clearly distinguishable in all MRI contrasts (Fig. 1, white arrows): T1w and T2w-FLAIR exhibit positive and negative contrasts, respectively. Thus, the T1w/T2w-FLAIR ratio amplified the contrast of the line of Gennari. Moreover, T1w/T2w-FLAIR images revealed bands of Baillarger in S3b in somatomotor cortex and in several areas within premotor, cingulate, insular, temporal and prefrontal cortices.Several cortical areas were clearly distinguishable (e.g., primary sensory areas) based on their distinctive laminar MRI profiles. For example, maps of laminar similarity for a selected vertex revealed a sharp transition between primary motor cortex (area 4) and S3a (Fig. 2A), coinciding with a cortical thickness change (Fig. 2H) and the HCP-style midthickness weighted T1w/T2w-FLAIR imaging gradients (Fig. 2F, G) at the fundus of the central sulcus. Moreover, sharp transitions between architectonic areas (1, 2, 3a, 3b, 5d and 5v) in the somatosensory cortex were also identifiable (Fig. 2B-E), despite representing one of the thinnest parts (1.2 - 1.7 mm) of the cerebral cortex (Fig. 2H; mean thickness 2.0 mm) with relatively strong curvature (Fig. 2I).
Discussion
Comparison between cortical laminar similarity (Fig. 2A-E) and the HCP-style midthickness weighted T1w/T2w-FLAIR imaging gradients (Fig. 2G) revealed coinciding transitions between several cortical areas thereby providing mutually supportive evidence. However, the transition between area 2 and dorsal portion of area 5 was less evident which may be ascribed to the very similar laminar myeloarchitecture between the two areas (7). In contrast, 5d and 5v exhibited distinct laminar MRI patterns (7,8,9). Moreover, laminar (dis-)similarity also suggests anterior-posterior division of motor cortex (Fig. 2A,J) (10) and several sharp cortical area boundaries in more lightly myelinated regions (e.g. see prefrontal cortex, Fig. 1D-F). We envision that the presented methodology may enable non-invasive investigations of laminar and areal features across multiple species. The presented results will also greatly benefit from our on-going efforts to directly compare T1w/T2w imaging with histological myeloarchitecture (6).Conclusions
Altogether, the presented methodology provides noninvasive means for quantitative comparisons of laminar myelin across cortical areas and potentially across species thereby advancing the pioneering work of classical anatomists.Acknowledgements
This research is partially supported by the program for Brain/MINDS and Brain/MINDS-beyond from Japan Agency for Medical Research and development, AMED (JP21dm037006, T.H.) and JSPS KAKENHI Grant Number (JP20K15945, J.A.A.).References
1. Vogt O. Die myeloarchitektonische Felderung des menschlichen Stirnhirns. J. Psychol. Neurol 1910;15, 221-232.
2. Nieuwenhuys, R. The myeloarchitectonic studies on the human cerebral cortex of the Vogt–Vogt school, and their significance for the interpretation of functional neuroimaging data. Brain Struct. Funct. 2013;218, 303–352.
3. Glasser M.F., and Van Essen D. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. Off. J. Soc. Neurosci. 2011;31, 11597–11616.
4. Glasser, M.F., et al. A multi-modal parcellation of human cerebral cortex. Nature 2016;536, 171–178.
5. Autio, J.A., et al. Towards HCP-Style macaque connectomes: 24-Channel 3T multi-array coil, MRI sequences and preprocessing. NeuroImage 2020;215, 116800.
6. Hayashi T., et al. The NonHuman Primate Neuroimaging & Neuroanatomy Project. Neuroimage 2021;229, 117726.
7. Mayer A., et al. Architectonic Mapping of Somatosensory Areas Involved in Skilled Forelimb Movements and Tool Use. J. Comp. Neurol. 2016;524,1399-1423.
8. Lewis J. and Van Essen D. Mapping of Architectonic Subdivisions in the Macaque Monkey, With Emphasis on Parieto-Occipital Cortex. J. Comp. Neurol. 2000;428, 79-111.
9. Pandya D. and Seltzer B. Intrinsic connections and architectonics of posterior parietal cortex in the rhesus monkey. J. Comp. Neurol. 1982;204, 196-210.
10. Rapan L., et al. Multimodal 3D atlas of the macaque monkey motor and premotor cortex. Neuroimage 2021;226, 117574.
Figures