0422
Evidence of a cortical hypoperfusion spreading following local ultrasound Blood Brain Barrier opening in the rat brain.1ToNIC, Toulouse NeuroImaging Center (UMR 1214 - Inserm / UPS), Toulouse, France, 2LabTAU, Inserm, Centre Léon Bérard, Université Lyon 1, Lyon, France, 3GIN, Grenoble Institut des Neurosciences, Inserm, Université Grenoble Alpes, Grenoble, France, 4CREFRE, Inserm, Université Toulouse III Paul Sabatier, ENVT, Toulouse, France
Synopsis
The use of ultrasound combined with microbubbles to open the blood-brain barrier is an increasingly common method in clinical and preclinical research. This work describes the effect of this BBB opening method on brain perfusion in rats, using pCASL sequence. Surprisingly, through a dynamic analysis, we observed a long time hypoperfusion spreading in the brain cortex following sonication. A similar phenomenon has been described in studies where cortical spreading depression (CSD) has been induced.
Introduction
The use of ultrasound combined with microbubbles to open the blood-brain barrier (BBB) is an increasingly common method in clinical and preclinical research [1]. However, its effects on cerebral perfusion have been poorly investigated. Our study aimed initially to test the reliability of the pseudo-continuous arterial spin labelling MRI sequence (pCASL) [2] to quantify cerebral blood flow (CBF) in the case of a disrupted BBB. The following work describes the effect of BBB opening on brain perfusion in rats, and examines the spatio-temporal evolution of this effect on the cortex of the US-exposed hemisphere.Material and methods
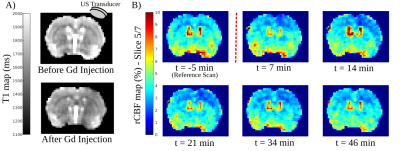
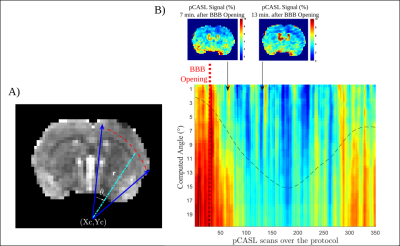
The protocol included 10 female Wistar rats, 3 of them served for control experiments. A catheter was placed on the tail vein of the anesthetized animals. Prior to sonication, animals were imaged in a 7T MRI scanner (Bruker Biospec 70/20 equipped with a volume transmit coil and a 2x2 elements surface receive coil) for preparation and optimization of pCASL acquisitions. Outside the magnet, the animals received an injection of 200μL of microbubbles (Sonovue, 8 µl/ml Sulfur hexafluoride) and their right hemisphere were exposed to unfocused ultrasounds (US). The flat transducer operates at a frequency of 1.13MHz, producing a theoretical 5mm beam (25ms pulse, every 1 second for 2 minutes) inducing an acoustic pressure of 0.3MPa. This acoustic pressure has been chosen in order to ensure safe and reversible BBB opening and to preserve tissues from any damage (no visible T1-W, T2-W and T2*-W image changes as criteria). A series of pCASL acquisitions (of spatial resolution: 320µm x 320µm x 1mm; TE = 15ms; TR = 2600ms; Labelling time = 2000ms, Post Labelling Delay = 300ms, Number repetition = 35, 7 coronal slices between +2mm and -4mm from anterior commissure) were then carried out every 4 min to assess Cerebral Blood Flow (CBF) after BBB opening. ADC maps were computed from the DTI-EPI Bruker protocol (TE = 20ms; TR = 2500ms; b~0 and 800s/mm² in 3 directions). Following the pCASL acquisitions, T1 maps were acquired, prior and after the injection of 300µL of Gado-DOTA (Dotarem, 0.5mmol/mL), using a Multi-Inversion Time recovery sequence (FAIR-EPI, TE = 13.5ms; TR = 10000ms, TI= (17) 20ms : ... : 9000ms). Three animals from the group underwent DSC acquisition during Gado-DOTA injection (TE = 10ms; TR = 500ms; Number of Repetitions: 300; Spatial Resolution: 390µmx390µmx1mm). All images were realigned to a T2-W reference image. rCBF (i.e. (Mcontrol-Mlabel)/Mcontrol)) and T1 maps were computed using MP3 software [3]. To investigate spatiotemporal dynamic of cortical rCBF, we converted the cartesian voxel coordinates to polar coordinates with a predefined center and an angular resolution of 0.5°.Results
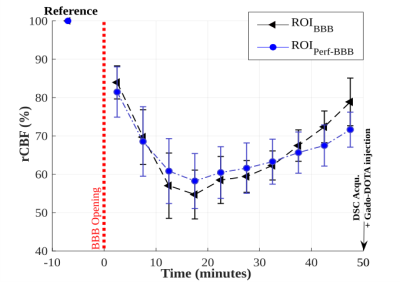
Local exposure to US combined with microbubbles induces the BBB opening as confirmed by the T1 decrease consecutive to Gado-DOTA extravasation in the beam path of US (Fig 1A). Modification of pCASL signal was detectable from the first acquisition following sonication (Fig 1B). rCBF and T1 maps display a mismatch between the volume with rCBF alteration and the one delimiting the BBB opening (Fig 1), albeit both volumes display similar rCBF evolution patterns (Fig 2). Thus, rCBF decreases up to 40 % within the 20 minutes post sonication then followed by a progressive recovery (Fig 2) confirmed by DSC analysis. A sharp observation of rCBF spatial evolution through the cortical area (Fig 1) shows an expansion through all the cortex. Quantitative approach using polar coordinates (Fig 3) demonstrates that the rCBF decrease propagates starting from an area located within the sonication beam.Discussion and Conclusion
Topically applied ultrasound to the rat brain through the skull is a useful method to perform BBB opening, especially to evaluate new oncotherapy in preclinical studies. Our observations confirm that pCASL signal is reliable to measure rCBF and not significantly affected by BBB disruption in agreement with the generalized model assuming a rapid water exchange [3]. However, despite caution taken to determine the minimum ultrasound power required to perform BBB opening, this method may induce a significant decrease in cortical perfusion. One hypothesis is a local myogenic response to a transmural pressure increase (i.e., the pressure across the vessel wall) occurring during the sonication time in presence of microbubbles [4]. However, the spatial-temporal dynamic of rCBF described in this work is similar to hypoperfusion observed in previous studies when cortical spreading depression (CSD) was induced [5]. Indeed, studies with CSD induced by a mechanical or an electrical stimulus [5,6] led to a depolarization followed by a depressed cortical activity associated with a long-lasting vasoconstriction.Acknowledgements
The ENI-CREFRE (Dr. C. Pestourie, Dr. J. Piraquive) and CREFRE animal experiment facilities (C. Baudelin) are gratefully acknowledged.References
[1] Hirschler L, Debacker CS, Voiron J, Köhler S, Warnking JM, Barbier EL. Interpulse phase corrections for unbalanced pseudo-continuous arterial spin labeling at high magnetic field. Magn Reson Med. 2018
[2] Brossard C, Montigon O, Boux F, Delphin A, Christen T, Barbier EL, Lemasson B. MP3: Medical Software for Processing Multi-Parametric Images Pipelines. Front Neuroinform. 2020
[3] Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998
[4] Lidington D, Kroetsch JT, Bolz SS. Cerebral artery myogenic reactivity: The next frontier in developing effective interventions for subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2018
[5] Guiou M, Sheth S, Nemoto M, Walker M, Pouratian N, Ba A, Toga AW. Cortical spreading depression produces long-term disruption of activity-related changes in cerebral blood volume and neurovascular coupling. J Biomed Opt. 2005
[6] Sato S, Kawauchi S, Okuda W, Nishidate I, Nawashiro H, Tsumatori G. Real-time optical diagnosis of the rat brain exposed to a laser-induced shock wave: observation of spreading depolarization, vasoconstriction and hypoxemia-oligemia. PLoS One. 2014
Figures