0417
Longitudinal simultaneous cortex wide Ca2+ imaging and whole brain functional magnetic resonance imaging in awake mice across the lifespan1Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Biomedical Engineering, Yale University, New Haven, CT, United States, 3Neurology and Neuroscience,, Yale University, New Haven, CT, United States, 4Urology, Yale University, New Haven, CT, United States, 5Neurology and Neuroscience, Yale University, New Haven, CT, United States
Synopsis
There is a clear need for imaging protocols in awake animals. Yet, there are only a few approaches in mice, despite their usefulness as translational research subjects. We introduce the first protocol for longitudinal simultaneous fMRI and Ca2+ imaging in awake mice. The same animals are imaged four times (from 4 to 12 months of age) – twice under anesthesia, and twice awake. Our framework includes two acclimation protocols: an initial intensive training followed by a ‘refresher' course. We find a marked improvement in motion between the two awake sessions. Implications for future dual-imaging experiments in awake mice are considered.
Introduction
Most mouse fMRI data are collected under anesthesia. Yet, there is evidence that anesthetics alter brain activity fundamentally – through modulating hemodynamic and neurovascular coupling1,2 – and globally – through changing the functional landscape of the brain3,4. This evidence highlights a need to conduct experiments in awake animals. During fMRI, the scanner emits noise >100dB. Movement of the head and body is prohibited as these cause artifacts. Both noise and immobilization are stress-inducing which biases brain activity towards a state of high alert. Emerging work in awake mice attempts to minimize stress and motion by implementing acclimation protocols5-13. Unfortunately, lower-order mammals like mice are difficult to accustom to awake fMRI. Yet, mice are attractive research subjects because of their small size (easier to image at higher field strengths), fast breeding cycles (low cost), and massively manipulatable genome (hundreds of disease models and tools that are genetically introduced - e.g., fluorescence markers). We present a protocol for longitudinal simultaneous fMRI and Ca2+ imaging in awake mice. We include four imaging time points: two under anesthesia, and two awake (anesthesia-naïve) spanning 3-12 months of age (moa). Here, we examine motion in both modalities and the longevity of the head-plate implant.Methods
For Ca2+ imaging and immobilization, mice (N=25) expressing Ca2+ indicators pan-neuronally underwent skull thinning and optical-window implantation at 3moa (Figure 1a). At 4moa and 6moa, dual-imaging data (fMRI and Ca2+ imaging) were acquired under isoflurane (ses-1 and ses-2; Figure 1b). Following ses-2, mice were split into ‘trained’ (N=9) and ‘control’ (N=6) groups. Control mice were imaged at 9moa (ses-3_control) and 12moa (ses-4_control) under isoflurane. Mice in the training group were, instead, acclimated to awake imaging prior to ses-3 where they were imaged awake (ses-3_awake). Prior to ses-4, trained mice were ‘reminded’ using a truncated protocol then imaged a second time whilst awake (ses-4_awake).Awake acclimation prior to ses-3. Stressors were introduced sequentially, and exposure (intensity and duration) was increased incrementally. After 4 days of handling, mice were exposed to three stressors over 10 days (Figure 2) in a mock-scanner: (1) restraint – head fixation and body suspended in a ‘hammock-like’ bed5,7,12, (2) exposure to LED lights - for Ca2+ recording, and (3) MRI noise. Two days of in-scanner dual-imaging followed.
Awake acclimation prior to ses-4. After ses-3, animals had 3 months of rest. Before undergoing the second awake session (ses-4_awake), we implemented a 2-day mock-scanner ‘refresher’ protocol. All stressors were reintroduced at full intensity and duration. Three days of in-scanner dual-imaging followed (Figure 2a, far right). Mice were imaged on an 11.7T magnet (Bruker). FMRI data were acquired using a gradient-echo, echo-planar-imaging sequence with a repetition time of 1.8s, and echo time of 10.5ms. Data were collected across 25 slices at 0.31×0.31×0.31mm3. Each run had 334 repetitions (10-minutes). We acquired three runs per session. Simultaneous Ca2+ data (1.4cm2 at 0.25x0.25μm2) were acquired at an effective 10Hz as described previously14. FMRI data were motion-corrected with 6 rigid parameters estimated as frame-to-frame divergence (FD) from the 150th frame of each run. Ca2+ data were motion-corrected, and all preprocessing steps were carried out as described previously14.
Results
Bodyweight was used to assess stress. Awake mice showed no signs of weight loss nor a difference from control animals (Figure 3). FMRI FD is visualized per volume over time (Figure 4a). A ‘high motion’ threshold of >0.1mm was adopted (Figure 4b). Anesthetized data showed low motion, as expected (Figure 4a, left), with 9% of frames >0.1mm FD (Figure 4b, far left). The first awake imaging session (ses-3_awake) showed motion spikes typical of awake fMRI, with 18% of frames >0.1mm FD. This success rate is in agreement with previous reports9,10,13,15,16. Encouragingly, the second awake imaging session (ses-4_awake) showed a marked decrease in motion amplitude and spike frequency (Figure 4a, right), with only 9% of frames >0.1mm FD (the same as our anesthetized data). Curiously, motion in the Ca2+ images showed no differences between awake and anesthetized conditions (Figure 4c), and low motion estimates overall. Since these data are in 2-dimensions (imaged from above), this confirms little motion in the X-Y plane. This holds for imaging sessions with high motion in the fMRI data. The integrity of the head-implant (for cortical optical access, and immobilization) is preserved throughout the 9 months that our longitudinal protocol spans (Figure 1b).Discussion
We present the first longitudinal, simultaneous Ca2+ and fMRI awake imaging protocol. Following 10-days of acclimation, which is among the longest reported5-13, we observe typical levels of motion in our awake fMRI data. This is encouraging given the added stress of the LED light required for Ca2+ imaging. Following a 3-month break, and a ‘refresher’, we see a reduction in motion – comparable to anesthetized levels – during our second awake dual-imaging session. This should encourage at least two awake imaging sessions in future studies. Our simultaneously acquired Ca2+ data reveals low motion in the X-Y plane during all sessions. This indicates that motion in fMRI data is likely restricted to Z or caused by B0 inhomogeneities from body movement. This finding will guide improvements to body restraint and B0 correction, although we find body suspension helps preserve the head-plate implant remarkably well.Acknowledgements
Drs. Joel Greenwood and Omer Mano from Neurotechnology Core and Dr. Peter Brown from Magnetic Resonance Research Center Yale University for their technological expertise. Yale Alzheimer’s Disease Research Center Scholar’s Award Funding.References
1. Rungta RL, Osmanski BF, Boido D, Tanter M, Charpak S. Light controls cerebral blood flow in naive animals. Nat Commun. 2017 Jan 31;8:14191.
2. Slupe AM, Kirsch JR. Effects of anesthesia on cerebral blood flow, metabolism, and neuroprotection. J Cereb Blood Flow Metab. 2018 Dec;38(12):2192-2208.
3. Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015 Jan 20;112(3):887-92.
4. Demertzi A, Tagliazucchi E, Dehaene S, Deco G, Barttfeld P, Raimondo F, Martial C, Fernández-Espejo D, Rohaut B, Voss HU, Schiff ND, Owen AM, Laureys S, Naccache L, Sitt JD. Human consciousness is supported by dynamic complex patterns of brain signal coordination. Sci Adv. 2019 Feb 6;5(2):eaat7603.
5. Yoshida K, Mimura Y, Ishihara R, Nishida H, Komaki Y, Minakuchi T, Tsurugizawa T, Mimura M, Okano H, Tanaka KF, Takata N. Physiological effects of a habituation procedure for functional MRI in awake mice using a cryogenic radiofrequency probe. J Neurosci Methods. 2016 Dec 1;274:38-48.
6. Jonckers E, Delgado y Palacios R, Shah D, Guglielmetti C, Verhoye M, Van der Linden A. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn Reson Med. 2014 Oct;72(4):1103-12.
7. Matsubayashi K, Nagoshi N, Komaki Y, Kojima K, Shinozaki M, Tsuji O, Iwanami A, Ishihara R, Takata N, Matsumoto M, Mimura M, Okano H, Nakamura M. Assessing cortical plasticity after spinal cord injury by using resting-state functional magnetic resonance imaging in awake adult mice. Sci Rep. 2018 Sep 26;8(1):14406.
8. Chen X, Tong C, Han Z, Zhang K, Bo B, Feng Y, Liang Z. Sensory evoked fMRI paradigms in awake mice. Neuroimage. 2020 Jan 1;204:116242.
9. Harris AP, Lennen RJ, Marshall I, Jansen MA, Pernet CR, Brydges NM, Duguid IC, Holmes MC. Imaging learned fear circuitry in awake mice using fMRI. Eur J Neurosci. 2015 Sep;42(5):2125-34.
10. Madularu D, Mathieu AP, Kumaragamage C, Reynolds LM, Near J, Flores C, Rajah MN. A non-invasive restraining system for awake mouse imaging. J Neurosci Methods. 2017 Aug 1;287:53-57.
11. Tsurugizawa T, Tamada K, Ono N, Karakawa S, Kodama Y, Debacker C, Hata J, Okano H, Kitamura A, Zalesky A, Takumi T. Awake functional MRI detects neural circuit dysfunction in a mouse model of autism. Sci Adv. 2020 Feb 5;6(6):eaav4520.
12. Takata N, Sugiura Y, Yoshida K, Koizumi M, Hiroshi N, Honda K, Yano R, Komaki Y, Matsui K, Suematsu M, Mimura M, Okano H, Tanaka KF. Optogenetic astrocyte activation evokes BOLD fMRI response with oxygen consumption without neuronal activity modulation. Glia. 2018 Sep;66(9):2013-2023.
13. Dinh TNA, Jung WB, Shim HJ, Kim SG. Characteristics of fMRI responses to visual stimulation in anesthetized vs. awake mice. Neuroimage. 2021 Feb 1;226:117542.
14. Lake EMR, Ge X, Shen X, Herman P, Hyder F, Cardin JA, Higley MJ, Scheinost D, Papademetris X, Crair MC, Constable RT. Simultaneous cortex-wide fluorescence Ca2+ imaging and whole-brain fMRI. Nat Methods. 2020 Dec;17(12):1262-1271.
15. Han Z, Chen W, Chen X, Zhang K, Tong C, Zhang X, Li CT, Liang Z. Awake and behaving mouse fMRI during Go/No-Go task. Neuroimage. 2019 Mar;188:733-742.
16. Desjardins M, Kılıç K, Thunemann M, Mateo C, Holland D, Ferri CGL, Cremonesi JA, Li B, Cheng Q, Weldy KL, Saisan PA, Kleinfeld D, Komiyama T, Liu TT, Bussell R, Wong EC, Scadeng M, Dunn AK, Boas DA, Sakadžić S, Mandeville JB, Buxton RB, Dale AM, Devor A. Awake Mouse Imaging: From Two-Photon Microscopy to Blood Oxygen Level-Dependent Functional Magnetic Resonance Imaging. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019 Jun;4(6):533-542.
Figures
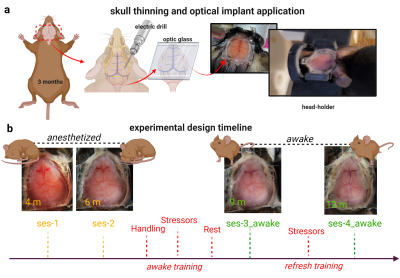
Figure 1| Surgical preparation, and dual-imaging timeline.
At 3moa, mice undergo scalp resection and skull-thinning prior to placement of a glass head-plate. This procedure optically exposes the cortex for Ca2+ imaging and gives purchase to secure the head during dual imaging. Photographs of the preparation are shown (a). An experimental timeline for dual-image acquisition is shown. Included are four dual imaging time points as well as both our acclimation protocols. Photographs from one mouse are shown at each time point to exemplify the longevity of the head-implant (b).
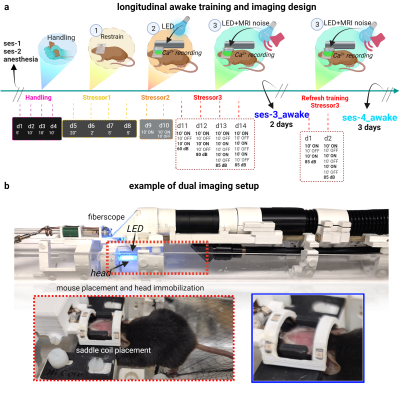
Figure 2| Acclimation for awake dual-imaging.
Timeline for acclimation (a). After ses-1&2, mice undergo 14-days of training in a mock-scanner where stressors (handling, restraint, light, and MRI noise) are introduced sequentially at increasing intensity/duration. After training, mice undergo 2-days of dual-imaging (ses-3_awake). 3 months later, mice undergo a 2-day ‘refresher’ where stressors are reintroduced at full intensity. After that, mice undergo 3-days of dual-imaging (ses-4_awake). Pictures of the ‘hammock’-bed, head-coil, fiberscope (for Ca2+ imaging) (b).
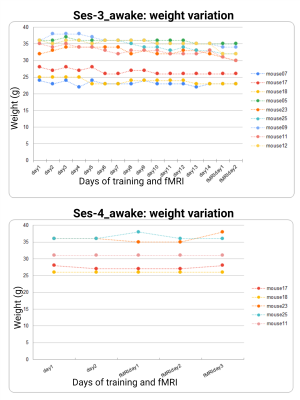
Figure 3| Weight measurements collected during acclimation and awake dual imaging.
Weight (in grams, g) is reported for all mice that undergo acclimation training and awake dual-imaging sessions. Training days in the mock-scanner environment are denoted as ‘day#’. Imaging days in the scanner are denoted as ‘fMRIday#’. Ses-3_awake data (full acclimation protocol) is plotted on the left, ses-4_awake data (the ‘refresher’) is plotted on the right. All animals showed no signs of weight loss throughout the study. No animals were excluded during either acclimation protocol.
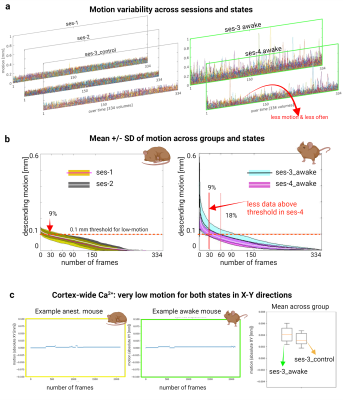
Figure 4| Dual-imaging motion results.
FMRI FD is calculated per run (3 runs per session) and displayed over time. Each run is 10mins (334 volumes). Anesthetized data are plotted on the left, awake data are plotted on the right (a). Note the decrease in motion between awake sessions 3&4. Sorted mean and SD of fMRI FD (b). Between the two awake sessions (right), there is a marked decrease in the number of frames >0.1mm FD – reaching the same fraction as anesthetized data (left). Ca2+ imaging shows very low motion in the X-Y plane in all sessions. Examples on the left, mean across mice on the right.