0416
Insights into Communicating Congenital Hydrocephalus on Neurodevelopment in a Mouse Model of Ciliopathy1Developmental Biology, University of Pittsburgh, Pittsburgh, PA, United States, 2Radiology, Children's Hospital of Pittsburgh, Pittsburgh, PA, United States
Synopsis
Mice homozygous to the partial loss-of-function Dnah5 allele, a component in the ependymal motile cilia, developed congenital hydrocephalus. Volumetric analysis showed enlarged aqueduct, indicating that the hydrocephalus was not caused by collapsed aqueduct, but rather by altered cerebrospinal fluid homeostasis. Furthermore, specific brain regions displayed dysplasia, including hippocampus, olfactory bulb and cerebellum. Diffusion tractography followed by topology analysis showed altered neuronal network organization in these brain areas. Our study suggests that ependymal cilia may play a crucial role in grey matter and white matter development and neuronal network organization.
INTRODUCTION:
Congenital hydrocephalus, characterized by enlarged ventricles and excessive cerebrospinal fluid (CSF) at birth, is a common neurological disorder with an estimated incident of 1-3 per 1000 live births [1] and is often fatal if left untreated. The pathophysiology of poor neurodevelopmental outcome of congenital hydrocephalus patients is thought to be due to enlarged ventricles that lead to mechanical injury of the brain. A new model is suggested by growing findings on ependymal motile cilia. There is an increasing appreciation that hydrocephalus can occur in patients and mouse models with ciliary defects[1-3]. In the healthy brain, orchestrated ependymal cilia beating generates a network of directional flow channels, a tightly regulated near-wall transport system for trafficking signaling molecules, chemokines, nutrients, and toxic waste for neurons, glia, and stem cells at precise locations with specific timing [4]. This pattern of cilia-driven near-wall CSF flow network is conserved across species. Ciliary defects are thought to lead to hydrocephalus by damaged ependymal surface causing collapse of the cerebral aqueduct wall resulting in aqueductal stenosis and altered CSF flow [5]. We hypothesized that ependymal ciliary signaling instead of aqueductal narrowing is important for CSF homeostasis and neurodevelopment. We tested this hypothesis in a genetic mouse model of ependymal motile ciliary gene Dnah5.METHODS:
Animal model: Dnah5 (Dynein Axonemal Heavy Chain 5) encodes a component of the axonemal heavy chain dynein protein complex for motile cilia. The partial lost-of-function Dnah5 allele, in homozygous mice, resulted in partially functional ependymal cilia that can beat but with reduced frequency and an altered near-wall CSF flow network.In vivo volumetric MRI: T2WT fast spin echo MRI was acquired at a preclinical 7-Tesla MRI (Bruker BioSpec USR 70/30) with a 35-mm quadrature volume coil, 1.5% isoflurane anesthesia, with the following parameters: in-plane resolution = 66 μm, SLTH = 0.5 mm, TE = 12.347 msec, RARE factor = 8, effective TE = 24.69 msec, and TR = 1500 msec. ITK-SNAP was used for volumetric analysis for brain regions and CSF.
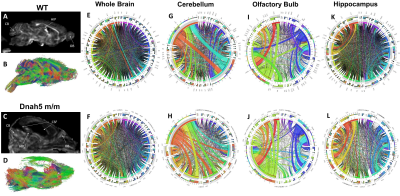
Diffusion tractography and neuronal network analysis: Mouse brains were subjected to diffusion MRI with isotropic 156 μm3 resolution, 30 diffusion directions, δ = 4msec, Δ = 8 msec, b = 1200 s/mm2. Neuronal fiber tracking without assigning any region of interest (ROI) or region of avoidance (ROA) were conducted using generalized deterministic fiber tracking with 100,000 seeding points using the open source DSI Studio with a q-space diffeomorphic reconstruction method. 3D T2WT anatomical imaging with 78 μm3 isotropic resolution is used to register the diffusion MRI of the same brain space to the Allen brain atlas to parcellate into 36 brain regions. Connectivity matrices counting the connecting tracts between brain regions, graph theoretical analysis, and diffusion parameters were calculated using DSI studios to form connectograms.
RESULTS:
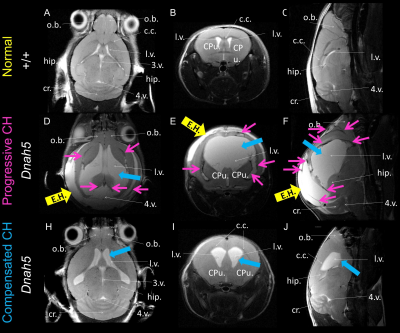
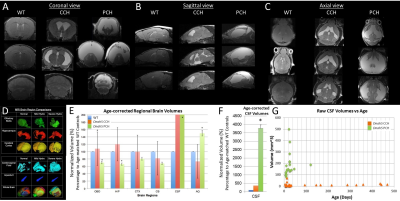
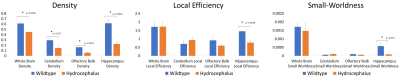
Most of mice homozygous to the partial loss-of-function Dnah5 allele developed progressive communicating hydrocephalus (PCH) by 3 to 4 weeks of age (Fig.1 D-F) with increased CSF volume and external hydrocephalus (E.H.). A small fraction of homozygous mice had the hydrocephalus spontaneous halted (Fig.1 H-J) compensated communicating hydrocephalus (CCH). Contrary to the previous hypothesis[6] that in Dnah5 mutant mice, the lack of ependymal flow owing to immotile ependymal cilia causes aqueductal narrowing, our volumetric analysis followed by in vivo anatomical MRI (Fig.2A-C) showed that our partial loss-of-function Dnah5 mice had larger or same-size aqueduct as the age-matched WT controls (Fig.2DE), indicating that Dnah5 mutant mice displayed CH with no aqueductal stenosis or obstruction. Mutant mice with PCH also showed dysplasia in specific subcortical regions, including hippocampus, olfactory bulb and cerebellum. Despite identical genotypes, mutant mice with PCH developed very severe CH within 1-2 months of age (Fig.2G green), whereas the CSF volumes of mutant mice with CCH remained relatively constant across lifespan (Fig.2G orange). Diffusion tractography (Fig.3B, D) followed by neuronal network analysis (Fig.3E-L) found that Dnah5 mutant mice displayed altered subcortical network in hippocampus (Fig.3KL), olfactory bulb (Fig.3IJ) and cerebellum (Fig.3EF), the same brain regions with dysplasia, with reduced density, local efficiency, and small-worldness (Fig.4).CONCLUSION:
Our study in the partial loss-of-function Dnah5 mutant mice suggested that congenital hydrocephalus is not due to anatomic narrowing of the aqueductal but, instead, functional altered CSF homeostasis. In addition, ependymal cilia may play a crucial role in multi-modal structural subcortical dysmaturation that is associated with hydrocephalus.Acknowledgements
MSC and YLW are supported by funding from NIH-R21-EB023507, AHA-18CDA34140024, and DoD-W81XWH1810070.References
1. Carter, C.S., et al., Abnormal development of NG2+PDGFR-alpha+ neural progenitor cells leads to neonatal hydrocephalus in a ciliopathy mouse model. Nat Med, 2012. 18(12): p. 1797-804.
2. Lee, L., Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res, 2013. 91(9): p. 1117-32.
3. Ibanez-Tallon, I., et al., Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet, 2004. 13(18): p. 2133-41.
4. Faubel, R., et al., Cilia-based flow network in the brain ventricles. Science, 2016. 353(6295): p. 176-8.
5. Strahle, J., et al., Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res, 2012. 3(Suppl 1): p. 25-38.
6. Fliegauf, M., T. Benzing, and H. Omran, When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol, 2007. 8(11): p. 880-93.
Figures