0415
Translational exploration of dynamic functional connectivity changes in Alzheimer’s disease patients and Thy-Tau22 mice at the prodromal stage1ICube Laboratory UMR 7357, Strasbourg University and CNRS, Strasbourg, France, 2Centre Mémoire de Ressources et de Recherche (CM2R), Strasbourg University Hospitals, Neurology and Geriatrics units, Strasbourg, France, 3Department of Biophysics and Nuclear Medicine, Strasbourg University Hospitals, Strasbourg, France
Synopsis
Between-species comparisons become essential to validate findings from animal models. In case of Alzheimer’s disease (AD) especially important is to evaluate their predictive potential for the sporadic form and to assess their relevance for the early stages of pathology. Therefore, we used dynamic resting-state functional connectivity to investigate and comparatively assess changes in early AD patients and prodromal Thy-Tau22 mice, a mouse model of tauopathy. We highlighted between-species similarities showing less dynamic communication of brain areas in diseased groups than in controls, and comparable modifications of memory and default mode-like networks in early AD and prodromal Thy-Tau22 mice.
Introduction
Exploring cerebral functional connectivity in mice models of AD offers a unique opportunity to highlight early changes in brain communication, even before the emergence of cognitive decline. It represents a crucial tool to identify potential vulnerable networks translatable to Humans, especially at prodromal stage. Between-species comparisons become therefore essential to validate findings from animal models, usually only partly reflecting the pathology in Human. We aimed to explore the translational potential of Thy-Tau22, a mouse model of Alzheimer’s disease (AD) expressing tauopathy at the prodromal stage of the disease. We conducted similar dynamic functional analyses in prodromal AD patients and early onset Thy-Tau 22 mice, to investigate and characterize homologue and specific alterations between species.Methods
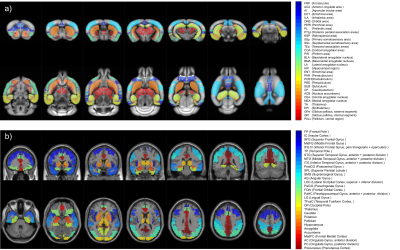
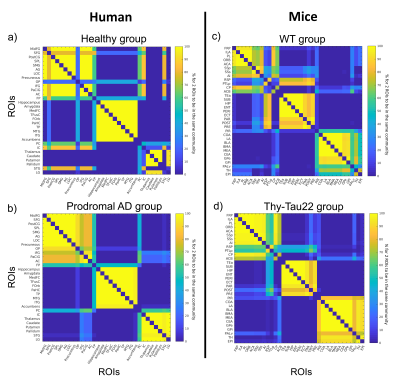
32 prodromal AD patients (MMSE score >24) compared to 19 healthy elderly subjects, and 16 Thy-Tau22 mice compared to 13 wild-type (WT) mice, at an age when no memory impairment is yet detected in transgenic mice1, were included in this study. Animal imaging was performed on a Bruker 7T animal scanner, under anesthesia (medetomidine) by s.c. infusion of 0.3mg MD/kg-Body-Weight/hour. We acquired T2*-weighted (GE) echo planar images covering the whole brain, 500 volumes (16min 40 sec), TE/TR = 15/2000ms, and resolution = 0.14×0.22x0.4mm. MRI in humans was performed on a Siemens Verio 3T scanner with a 32-channel head coil. Whole-brain T2*-weighted (GE) echo planar images were acquired with 120 volumes (6min), TE/TR = 21/3000ms, and 4mm isotropic voxels. Similar preprocessing steps were conducted in patients and mice, including bias intensity correction, motion correction, normalization to the Allen Brain Atlas for mice and to MNI space using the DARTEL approach for Humans, and smoothing and BOLD-frequency filtering. Time courses were extracted for 33 regions of interests (ROIs) for mice and 31 ROIs for Humans (Figure 1). These regions were selected for their known involvement in AD and the existence of a potential functional homologue ROI between the 2 studied species. Dynamic resting-state functional connectivity (DFC) analyses were then carried out with a sliding-window approach (overlapping tappered window of 100s) leading to 352 signed-weighted correlation matrices per mouse and 87 per human subject. For each temporal window, we calculated the mean correlation matrices of each group, and used a generalization of the Louvain community detection algorithm2 to subdivide the 33-nodes animal network (resp. 31-nodes human network) into communities, i.e. non-overlapping groups of nodes that maximizes the number of within-group connections and minimizes the number of between-group connections. We obtained 352 (resp. 87) optimal community subdivisions, also called partitions, for the pathological and control groups in both species. Partitions were then aggregated to create an agreement matrix, indicating the percentage of time during acquisition any two ROIs were assigned to the same community. Partitions and agreement matrices make possible quantitative and qualitative comparisons of DFC changes detected in humans and mice.Results
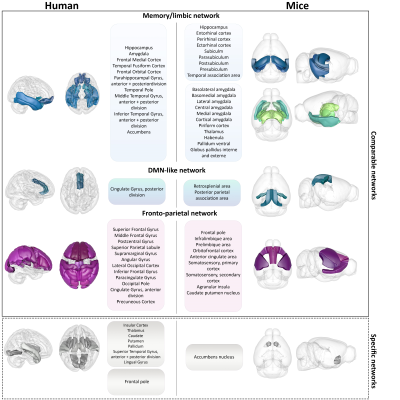
Agreement matrices of DFC, show higher probabilities for 2 given ROIs to belong and remain in the same functional module during the whole acquisition time, in pathological groups compared to control groups, in both humans and mice (Figure 1). Partition analyses (Figure 2) also revealed that diseased groups show a less flexible functional connectivity between ROIs when compared to respective controls. Further specific analysis of Thy-Tau22 and early-AD patients’ DFC, demonstrated comparable functional modules extracted from the agreement matrices in both species (Figure 4). For instance, homologue ROIs were included within memory and limbic networks, as well as in a default-mode-network (DMN)-like module, in both Thy-Tau22 mice and AD patients. In addition, one network resembling the fronto-parietal network in humans was partly found in Thy-Tau22 mice. Results also showed species-specific functional communities, as illustrated by a network including basal ganglia nuclei and the insula, only detected in patients.Discussion
In this study, we found DFC similarities between prodromal Thy-Tau22 mice and AD patients, showing a higher probability for functional networks to remain in the same connectivity state over time, and fewer transitions between connectivity states in pathology groups compared to their controls. These results suggest a functional hyperconnectivity in mice and human diseased groups, possibly subtended by compensatory mechanisms occurring at early stages of the disease, and supported by literature in both species3-7. We highlighted DFC similarities between transgenic mice and early AD patients in networks known to be particularly affected in AD, such as a memory/limbic and a DMN-like network. DFC modules were less comparable in networks involving less disrupted areas in early AD, such as basal ganglia nuclei.Conclusion
In this preliminary study we explored for the first time between-species homologies using DFC. We took advantage of the unique opportunity to investigate early stages of AD using a transgenic animal model and early AD patients. We highlighted DFC similarities in early Thy-Tau22 mice and AD patients, demonstrating the translational potential of our approach. The results are based on comparable processing and analysis methods, hold the promise for further methodological developments allowing the investigation of similar and/or specific features between animal models and patients at whole brain level and in early phase of the disease. Such approaches are essential to increase the translational potential between preclinical and clinical research and find synergistic biomarkers of early stages of the disease.Acknowledgements
The authors would like to acknowledge the High Performance Computing Center of the University of Strasbourg for supporting this work by providing scientific support and access to computing resources. Part of the computing resources were funded by the Equipex Equip@Meso project (Programme Investissements d'Avenir) and the CPER Alsacalcul/Big Data.References
1. Hutchison, R. M. et al. Dynamic functional connectivity: Promise, issues, and interpretations. NeuroImage 80, 360–378 (2013).
2. Rubinov, M. & Sporns, O. Weight-conserving characterization of complex functional brain networks. NeuroImage 56, 2068–2079 (2011).
3. Degiorgis, L. et al. Brain network remodelling reflects tau-related pathology prior to memory deficits in Thy-Tau22 mice. Brain 143, 3748–3762 (2020).
4. Dickerson, B. C. et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65, 404–411 (2005).
5. Bakker, A. et al. Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron 74, 467–474 (2012).
6. Shah, D. et al. Early pathologic amyloid induces hypersynchrony of BOLD resting-state networks in transgenic mice and provides an early therapeutic window before amyloid plaque deposition. Alzheimer’s & Dementia 12, 964–976 (2016).
7. Latif-Hernandez, A. et al. Subtle behavioral changes and increased prefrontal-hippocampal network synchronicity in APPNL−G−F mice before prominent plaque deposition. Behavioural Brain Research 364, 431–441 (2019).
Figures