0411
Deep Learning Reconstruction-Enabled 2D and 3D MR Neurography1Hospital for Special Surgery, New York, NY, United States, 2GE Healthcare, Chicago, IL, United States
Synopsis
Deep learning reconstruction (DLRecon) was applied to shorter acquisition 2D Dixon and 3D short-tau-inversion recovery (STIR) brachial plexus MR neurography (MRN) sequences and were compared both qualitatively and quantitatively to standard clinical sequences. DLRecon 2D and 3D images demonstrated similar quality to standard images. DLRecon may facilitate implementation of shorter MRN protocols, thereby helping streamline MRN’s incorporation into busy clinical practices and providing the option of acquiring additional imaging planes.
Introduction
MR neurography (MRN) primarily uses T2-weighted (T2w) pulse sequences(1), and is challenging to perform, as imaged nerves are often small, requiring millimeter to sub-millimeter spatial resolution. Multiple 2D and 3D imaging sequences with long scan times (5-9 minutes/sequence) are sometimes required to appreciate both cross-sectional and longitudinal nerve morphology and fascicular architecture. Longer acquisition times lead to increased motion artifact and need for repeated scans, especially in motion-prone chest wall regions around the brachial plexus. Respiratory-gating, while sometimes effective(2), requires patient compliance and additional scan time. Gadolinium contrast may additionally be required for vascular suppression(3). Consequently, MRN exams are frequently slow, laborious, and not streamlined, requiring verification of image quality and sequential determination by the monitoring radiologist as to the next appropriate sequence needed. Deep learning (DL) reconstruction techniques(4) have recently become commercially available for 2D fast/turbo-spin-echo (FSE/TSE) imaging on vendor platforms(5,6), and provide opportunities for faster scans by reducing noise and improving image sharpness. However, DL reconstruction techniques for 2D Dixon and 3D MRN have yet to be demonstrated. This is partly related to increased computational complexities for processing multi-echo and 3D-Fourier-transformed data, as well as increased hardware requirements.This study’s aim was to demonstrate the feasibility for faster, unilateral brachial plexus 2D Dixon and 3D MRN sequences using DL reconstruction. We hypothesized that DL-enabled faster sequences would provide similar image quality to longer, standard sequences.
Methods
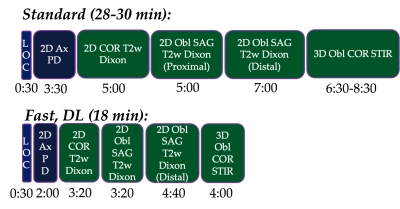
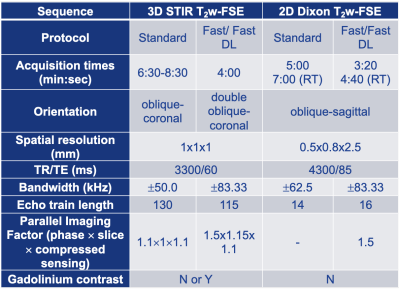
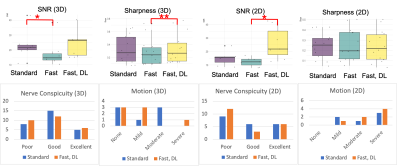
A total of 12 patients (4F/8M, mean age=53.7, range=16-83 years) undergoing standard, clinical brachial plexus MRN at 3T (Premier, GE Healthcare) over a two-week time period provided written consent for this prospective study under an IRB-approved protocol. Additional, ‘fast’ scans with identical spatial resolution were acquired depending on which ‘standard’ clinical sequence(s) were obtained: both or either 2D oblique-sagittal Dixon-T2w-FSE and/or 3D oblique-coronal STIR-T2w-FSE scans (Fig. 1 and Table 1). This provided a total of n=7 3D and n=7 2D paired comparisons. The ‘fast’ protocol included shorter scans (by ~36%) using a combination of higher receiver bandwidth and fewer phase-encoding steps and were first reconstructed without DL. DL reconstruction (DLRecon)(7) was modified to process either 2D multi-echo FSE data (for generating ‘water’ Dixon images) or 3D FSE data(8). k-space data underwent DLRecon using a separate workstation (iMac, 4.2Ghz Intel QuadCore i7, 32GB RAM), with ‘fast DL’ images returned to PACS (Sectra IDS7) for evaluation.For quantitative evaluation of nerve conspicuity, line profiles were drawn following a previously described method (9) to assess (i) sharpness of signal intensity slopes of C6 nerve root boundaries, and (ii) SNR of muscle within the same field-of-view (trapezius or deltoid). For (i), mean-normalized signal intensity slopes at C6 nerve root boundaries were calculated. For (ii), mean signal was divided by the standard deviation after linear fitting for signal non-uniformity. For qualitative evaluation, one musculoskeletal radiologist with 7 years’ dedicated MRN experience graded ‘standard’ and ‘fast DL’ side-by-side for nerve conspicuity (roots, trunks, divisions, cords) on a 3-point scale (poor, good, excellent), and bulk motion on a 4-point scale (none, mild, moderate, severe).
Statistical analyses were performed using paired, non-parametric Wilcoxon signed rank test for quantitative comparisons between ‘standard’, ‘fast’ and ‘fast DL’ with Bonferroni correction, and qualitative comparison between ‘standard and ‘fast DL’ only (α=0.05).
Results
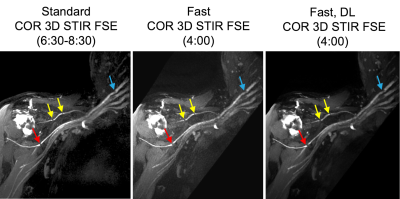
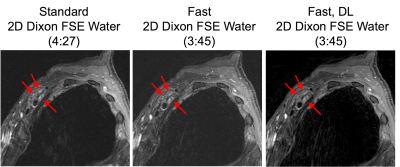
Abnormal MR findings were identified in 5/12 subjects: two with Parsonage Turner syndrome (Fig. 2); two with stretch injuries, and one with radiation-related neuropathy (Fig. 3). Quantitatively, SNR of 3D ‘fast’ was inferior to that of 3D ‘standard’ (by -5.4, p=0.015), and sharpness of 3D ‘fast DL’ was superior to 3D ‘fast’ by +0.060/pixel (p=0.0001) (Fig. 4). SNR of 2D ‘fast DL’ was superior to 2D ‘fast’ by +13.8 (p=0.016). All other quantitative and all qualitative comparisons were not significantly different.The range of scan time savings were 33-43% for 3D (mean=37%), and 24-35% for 2D (mean=29%). DLRecon processing times for 3D and 2D were 170 and 5.5 minutes, respectively.
Discussion
Quantitative and qualitative comparisons in T2-weighted MRN found that standard sequences and DL-reconstructed faster exams were of similar image quality, for both 2D Dixon-FSE and 3D STIR-FSE. These preliminary results suggest that a faster DL-enabled protocol may potentially replace currently optimized non-DL protocols. This may facilitate additional sequences and scan planes to be acquired, to better streamline MRN examinations.Patient motion between scans may have strongly biased image quality. As ‘fast’ scans were acquired after ‘standard’ in all comparisons motion may be more severe in ‘fast’ scans. More comparisons, with order of scans reversed, are needed. While this work targeted the most motion-sensitive exams of the brachial plexus protocol, comparison of additional scan planes and nerve anatomies would be needed. Furthermore, reconstruction times are currently long for clinical practice. Implementation of 3D DLRecon for an efficient clinical workflow would likely require use of GPUs. While this work did not qualitatively evaluate the same scans without and with DLRecon, previous evaluation in MRN (9) showed improved epineurium conspicuity and fascicular architecture despite slightly more conspicuous pulsation and ghosting artifacts.
Conclusion
Deep learning reconstruction in faster 2D multi-echo and 3D MRN can provide similar image quality to standard scans, to provide shorter exam times and streamlined MRN protocols.Acknowledgements
HSS receives institutional research support from GE Healthcare.References
1. Filler AG, Maravilla KR, Tsuruda JS. MR neurography and muscle MR imaging for image diagnosis of disorders affecting the peripheral nerves and musculature. Neurol Clin 2004;22:643 682 doi: 10.1016/j.ncl.2004.03.005.
2. Sneag DB, Mendapara P, Zhu JC, et al. Prospective respiratory triggering improves high-resolution brachial plexus MRI quality. J Magnetic Reson Imaging Jmri 2018;49:1723–1729 doi: 10.1002/jmri.26559.
3. Sneag DB, Daniels SP, Geannette C, et al. Post-Contrast 3D Inversion Recovery Magnetic Resonance Neurography for Evaluation of Branch Nerves of the Brachial Plexus. Eur J Radiol 2020;132:109304 doi: 10.1016/j.ejrad.2020.109304.
4. Hammernik K, Klatzer T, Kobler E, et al. Learning a variational network for reconstruction of accelerated MRI data. Magnet Reson Med 2018;79:3055–3071 doi: 10.1002/mrm.26977.
5. Velde N van der, Hassing HC, Bakker BJ, et al. Improvement of late gadolinium enhancement image quality using a deep learning–based reconstruction algorithm and its influence on myocardial scar quantification. Eur Radiol 2021;31:3846–3855 doi: 10.1007/s00330-020-07461-w.
6. Kidoh M, Shinoda K, Kitajima M, et al. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn Reson Med Sci 2020;19:195–206 doi: 10.2463/mrms.mp.2019-0018.
7. Lebel RM. Performance characterization of a novel deep learning-based MR image reconstruction pipeline. Arxiv 2020.
8. Sun S, Tan ET, Carrino J, et al. Evaluation of Deep-Learning Reconstructed High-Resolution 3D Lumbar Spine MRI to Improve Image Quality. Proc. ISMRM 2021.
9. Zochowski KC, Tan ET, Argentieri EC, et al. Improvement of peripheral nerve visualization using a deep learning-based MR reconstruction algorithm. Magn Reson Imaging 2022;85:186–192 doi: 10.1016/j.mri.2021.10.038.
Figures