0405
Skeletal muscle acetylcarnitine during submaximal exercise and recovery: Interleaved 1H/31P MRS 7T pilot study1Division of Endocrinology and Metabolism, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria, 2High-Field MR Center, Department of Biomedical Imaging and Image-Guided Therapy, Medical University of Vienna, Vienna, Austria, 3High-Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria, 4Christian Doppler Laboratory for Clinical Molecular MR Imaging (MOLIMA), Christian Doppler Laboratory, Vienna, Austria
Synopsis
The study aimed to explore the behavior of skeletal muscle acetylcarnitine and phosphocreatine during submaximal plantar flexion exercise using interleaved 1H/31P MRS at 7T. Acetylcarnitine decreased to steady state during the exercise, increased back to initial levels in early recovery period and started to decline during later phase of recovery. Studies including more volunteers and patients with broader range of metabolic conditions and physical fitness will be necessary for detailed quantitative analysis.
Introduction
Acetylcarnitine(AcC) plays an important role in fat metabolism and can be observed in skeletal muscle tissue at 2.13ppm by proton magnetic resonance spectroscopy(1H MRS)(1,2). Dynamic measurement of phosphocreatine(PCr) and inorganic phosphate(Pi) during and after exercise using 31P MRS(3) is an established method to non-invasively assess muscle energy metabolism and distinguish between different metabolic/pathologic states. It is already known that heavy workout leads to increased AcC concentrations immediately after the exercise challenge(4). However, the in vivo behavior of muscle AcC content during exercise with MRS was not shown. We therefore aimed to explore the behavior of AcC in more detail during rest, submaximal plantar flexion exercise and recovery in the human gastrocnemius muscle(GM) and relate to PCr and Pi evolution during a single experiment using interleaved 1H/31P MRS at 7T.Methods
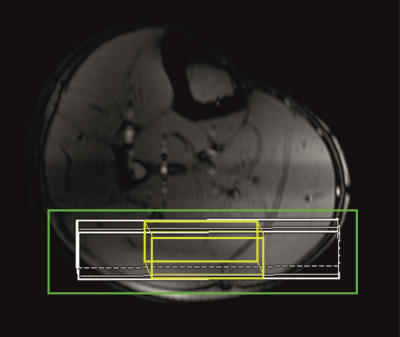
Six healthy, recreationally active volunteers (Age30±5years, BMI21.5±3.3kg/m2, sex4f/2m) participated in the study. All MR measurements were performed on a 7T whole-body MR system(Terra Dot Plus, Siemens Healthineers, Erlangen, Germany). A double-tuned surface coil transceiver array with two 1H channels(d = 17 cm, l = 12.5 cm) and three 31P channels(d = 15 cm, l = 10 cm), shaped to the anatomy of the human calf(5) was used to acquire spectra from the GM of the right leg. An MR-compatible ergometer(Trispect, Ergospect, Innsbruck, Austria) was used for plantar flexion exercise. Measurements were performed in the morning after an overnight fast in a single exercise-recovery session. Volunteers were lying in supine position with the right calf muscle placed on the double-tuned 1H/31P coil, on the ergometer, inside the 7T MR system. For volume of interest(VOI) positioning localizer MR images were acquired. The VOI for 1H MRS AcC detection(15x40x53 mm3) and slab for 31P MRS PCr and inoganic phosphate(Pi) detection(thickness of 18 mm) were carefully placed predominantly within the gastrocnemius medialis and lateralis muscles. The linewidth of water after shimming was in the range of 35–45 Hz, in magnitude spectra.Semi-LASER(6,7) localized 1H MR spectra(TE=300ms)(AcC) and slab-localized(DRESS)(8) 31P MR spectra(PCr, Pi) were acquired simultaneously in one interleaved exercise/recovery session(2 min rest, 15 min exercise at 30% of maximal voluntary contraction, 6 min recovery) with a TR of 6s, similarly as published(9). Within every TR volunteers were instructed to press the pedal twice between measurements, with noise of the spoiler gradients serving as audio cue, to ensure data acquisition in a relaxed state of the muscle. All spectra were analyzed with AMARES, using jMRUI v6.0 alpha. For absolute quantification of AcC, the fully relaxed water signal was measured separately(TR=6000ms, TE=30ms). The 1H MR spectra were averaged over one-minute periods(NA= 10) and AcC concentrations were calculated as described in(10) in mmol /L tissue volume units(further mmol/L only). The 31P MRS protocol yielded concentrations of PCr and Pi, depletion of PCr during the exercise, time constant or rate of PCr resynthesis during recovery(τPCr, VPCr), maximal rate of oxidative phosphorylation, i.e., mitochondrial capacity(Qmax), and time course of intracellular pH changes in skeletal muscle(11). Data are presented as means ± standard deviations.Results
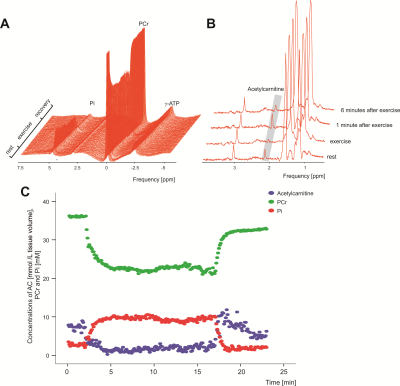
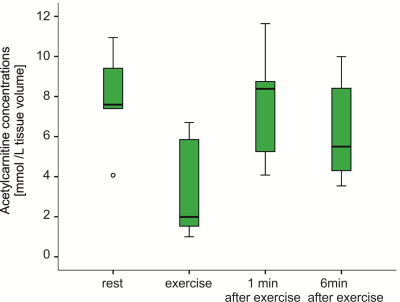
Chosen timing and the load of exercise protocol was well accepted by all volunteers included, and the time-resolved interleaved 1H/31P MRS acquisition yielded excellent data quality(Fig.2 C). As expected, PCr concentrations during exercise were decreasing, reached steady state and recovered towards steady state after exercise(Fig.2 A and C). Mean exercise related drop of PCr 27.5±4,7%; τPCr of 27.8±5,6s, VPCr of 0.34±0.08mM.s-1 and Qmax of 0.61±0.15mM.s-1 indicated that the exercise had been submaximal and aerobic. During the plantar flexion exercise period the AcC concentrations decreased from 7.28±2.69mmol/L to 3.33±2.59mmol/L. In the initial recovery period(1 min) AcC increased to 7.30±3.23mmol/L with a subsequent decrease to 6.06±2.92mmol/L in the last minute of the recovery period(Fig. 3).Conclusion
To our best knowledge, we are the first to show in vivo changes of skeletal muscle AcC during acute exercise and immediate exercise recovery with submaximal aerobic workload which can be tolerated by broad range of volunteer and patient populations. In correspondence to previous high-intensity exercise protocols(4,12) we could observe an increase of AcC in the early phase of recovery, followed by AcC wash-out in later recovery. The differences in the timing of peak AcC during recovery and the extent of wash-out may reflect the load and duration of previous exercise, as well as the physiological differences between muscles groups studied. Even-though we could observe qualitatively similar trends between AcC and PCr during the exercise and early recovery, we could not detect quantitative correlations between any measures of those two processes. Studies including volunteers and patients with higher numbers of subjects and a broader range of metabolic conditions and physical fitness will be necessary for detailed analysis.Acknowledgements
Colleagues at the High-Field MR Center, this study was supported by FWF (#KLI 904 to MKrss)References
1. Lindeboom L, et al. Long-echo time MR spectroscopy for skeletal muscle acetylcarnitine detection. J Clin Invest. 2014;124(11):4915–25.
2. Klepochová R, et al. Detection and Alterations of Acetylcarnitine in Human Skeletal. Invest Radiol. 2017;52(7):412–8.
3. Meyerspeer M, et al. 31 P magnetic resonance spectroscopy in skeletal muscle: Experts’ consensus recommendations. NMR Biomed. 2020;34(5).
4. Ren J, et al. Dynamic monitoring of carnitine and acetylcarnitine in the trimethylamine signal after exercise in human skeletal muscle by 7T 1 H-MRS. Magn Reson Med. 2013;69(1):7–17.
5. Goluch S, et al. A form-fitted three channel phosphorus 31P, two channel 1H transceiver coil array for calf muscle studies at 7 T. Magn Reson Med. 2015;73(6):2376–89.
6. Meyerspeer M, et al. Semi-LASER localized dynamic 31P magnetic resonance spectroscopy in exercising muscle at ultra-high magnetic field. Magn Reson Med. 2011;65(5):1207–15.
7. Scheenen TWJ, et al. Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Vol. 59, Magnetic Resonance in Medicine. 2008. p. 1–6.
8. Bottomley PA, Foster TB, Darrow RD. Depth-resolved surface-coil spectroscopy (DRESS) for in Vivo 1H, 31P, and 13C NMR. J Magn Reson. 1984;59(2):338–42.
9. Niess F, et al. Interleaved 31P MRS/1H ASL for analysis of metabolic and functional heterogeneity along human lower leg muscles at 7T. Magn Reson Med. 2020;83(6):1909–19.
10. Klepochová R, et al. Muscle‐Specific Relation of Acetylcarnitine and Intramyocellular Lipids to Chronic Hyperglycemia: A Pilot 3‐T 1 H MRS Study. Obesity. 2020;28(8):1405–11.
11. Valkovič L, et al. Skeletal muscle alkaline Pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Sciientific Reports, Nat. 2016;(December 2015):1–9.
12. Seiler SE, et al. Carnitine Acetyltransferase Mitigates Metabolic Inertia and Muscle Fatigue during Exercise. Cell Metab. 2015;22(1):65–76.
Figures