0402
Exploring Brain Connectivity in Ageing Using Explainable Deep Learning
Tamar van Asch1, Nathan De Jong1, Walter Backes1, Sebastian Köhler 2, Martin van Boxtel2, Miranda Schram3, and Jacobus Jansen1
1Radiology, Maastricht University Medical Center, Maastricht, Netherlands, 2Psychiatrie & Neuropsychologie, School for Mental Health and Neuroscience, Maastricht, Netherlands, 3Internal Medicine, Maastricht University, Maastricht, Netherlands
1Radiology, Maastricht University Medical Center, Maastricht, Netherlands, 2Psychiatrie & Neuropsychologie, School for Mental Health and Neuroscience, Maastricht, Netherlands, 3Internal Medicine, Maastricht University, Maastricht, Netherlands
Synopsis
There is a growing need for the understanding of the process of ageing and the ability to predict who is at risk of neurodegenerative diseases and mortality. This study aims to develop and train a convolutional neural network on structural brain connectivity data to predict age. dMRI is used to map the structural connectivity of the brain. The dataset comprises 3494 subjects from The Maastricht Study, a cohort study of individuals aged between 40 and 77 years. Brain age prediction on the test set resulted in a Pearson’s correlation coefficient of 0.70 and a mean absolute error of 5.1 years.
Introduction
There is a growing need to understand the ageing process, particularly as it relates to the brain and cognition. An individual’s brain health is often assessed by a biomarker called brain age. A large difference between one’s chronological age and brain age is an indicator of accelerated or decelerated ageing. 1 Consequently, the development of accurate brain age prediction models could lead to an early detection tool for better prediction of who is at risk of accelerated ageing and mortality due to neurodegenerative diseases such as Alzheimer’s disease.2 Also, brain age research can provide understanding of the positive effects of health characteristics and life experiences on brain ageing 6 such as physical exercise, years of education and practicing meditation. 3–5Brain age is typically estimated from structural MRI, based on gray matter volumes, using deep learning networks.6 This approach is effective; however age-related variations in structural and functional brain connectivity networks may contain additional information about ageing. These networks have been linked to both ageing and cognitive performance7, and can be derived from diffusion weighted (dMRI) and functional MR imaging (fMRI), respectively. The brain network can be represented by brain regions and their corresponding connections.8 These connections form highly complex 3D networks, which can be represented by a compact 2D matrix called a connectivity matrix (Figure 1). This complex data is difficult to assess in order to obtain neurobiologically meaningful measures, however explainable deep learning methods seek to identify the information that is most relevant to the predicted age.
The goal of this research is to gain a better understanding of the ageing process, and the role of connectivity networks in ageing. To do this, we develop and train a convolutional neural network to predict brain age from MR-derived brain connectivity data. We then examine the model using explainable deep learning methods to identify brain connectivity properties which are most predictive of age.
Methodology
Data were used from The Maastricht Study, an observational, prospective, population-based cohort study with extensive phenotyping of participants aged 40-77 years, enriched for type-2 diabetes. Cognitive test scores and 3T MRI (structural, d-MRI, and rs-fMRI) were available for n=4120 cognitively healthy participants (46.9% male, 17% with T2DM). Exclusion criteria were cognitive impairment (MMSE score <24 or >1.5SD below mean cognitive performance in memory, information processing speed and executive function) and high burden of cerebral small vessel disease (cSVD score >2), derived from white matter hyperintensity (WMH) load, presence of cerebral microbleeds and lacunar infarcts.Images were registered and segmented into 94 regions using the automatic anatomical labelling 2 (AAL2) atlas. Tractography and derivation of the connectome from MRI was performed using the diffusion MR Toolbox ExploreDTI version 4.8.6 and Brain Connectivity Toolbox in MATLAB Release 2016a. The edge weights of the connectome were derived from the tract volume and functional correlation between each region.8
The resulting connectivity matrices cannot be processed using a typical convolutional neural network (CNN), as regular convolution would undermine the topology of the matrix, because surrounding elements in the matrix are not per definition in close proximity in the brain. Therefore, a unique CNN architecture10 which preserved the topology of the connectivity matrix (Figure 2) was implemented to predict each individual’s brain age from their connectivity matrices.
The CNN learns which elements in the connectivity matrix are important for the prediction of age. In order to extract these elements, two explainable deep learning methods are implemented: sensitivity analysis and Integrated Gradients9,10, which map the attribution of each connection to the prediction score of age. This is visualized in an attribution map (Figure 3).
Results
Evaluation on an independent test set showed that the network could predict age with a mean absolute error of 5.1 years. The correlation between the chronological age and the predicted age was 0.70. The MAE is comparable with Chen et al.11, to our knowledge the only other report on brain age prediction using deep learning on dMRI derived features. The attribution map resulting from the explainable deep learning methods is shown from an axial (left) and coronal (right) point of view in Figures 3. Connections in the superior and medial frontal lobe have a large contribution to the prediction score.Discussion and Conclusion
The Last-in-last-out hypothesis suggests that ageing in the brain reverses the sequence of development.12 The hypothesis suggests that tracts that are developed last are more vulnerable to injury and decline in later life. White matter tracts that are relatively late to mature connect brain regions within one hemisphere, and are especially important for higher cognitive functions.13 Our results support findings of significant age-induced changes in the prefrontal cortex.12 The MFG was also found to contribute to the prediction of age in previous research.1Within the current study the feasibility of training on brain network connectivity data has been demonstrated, including a method to explain the input features that are contributing the most to the output score. This study could be further expanded to explore network differences associated with different neurodegenerative diseases, or identify modifiable risk factors which decelerate ageing and reduce dementia risk.
Acknowledgements
No acknowledgement found.References
- Bellantuono, L. et al. Predicting brain age with complex networks: From adolescence to adulthood. Neuroimage 225, 117458 (2021).
- Franke, K., Ziegler, G., Klöppel, S. & Gaser, C. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: Exploring the influence of various parameters. Neuroimage 50, 883–892 (2010).
- Cole, J. H. et al. Brain age predicts mortality. Mol. Psychiatry 23, 1385–1392 (2018).
- Steffener, J. et al. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol. Aging 40, 138–144 (2016).
- Luders, E., Cherbuin, N. & Gaser, C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. Neuroimage 134, 508–513 (2016).
- Franke, K. & Gaser, C. Ten years of brainage as a neuroimaging biomarker of brain aging: What insights have we gained? Front. Neurol. 10, (2019).
- Garcia-Cabello, E. et al. The Cognitive Connectome in Healthy Aging. Front. Aging Neurosci. 13, 1–15 (2021).
- Rubinov, M. & Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 52, 1059–1069 (2010).
- Simonyan, K., Vedaldi, A. & Zisserman, A. Deep inside convolutional networks: Visualising image classification models and saliency maps. 2nd Int. Conf. Learn. Represent. ICLR 2014 - Work. Track Proc. 1–8 (2014).
- Sundararajan, M., Taly, A. & Yan, Q. (Integrated Gradient)Axiomatic attribution for deep networks. 34th Int. Conf. Mach. Learn. ICML 2017 7, 5109–5118 (2017).
- Chen, C. Le et al. Generalization of diffusion magnetic resonance imaging–based brain age prediction model through transfer learning. Neuroimage 217, (2020).
- Raz, N. Ageing and the Brain. Encycl. Life Sci. (2003) doi:10.1038/npg.els.0003375.
- Lu, P. H. et al. Age-related slowing in cognitive processing speed is associated with myelin integrity in a very healthy elderly sample. J. Clin. Exp. Neuropsychol. 33, 1059–1068 (2011).
Figures
Figure 1: Visualization of the
construction of a brain network with the human brain as a graph network (left)
and its corresponding weighted adjacency or connectivity matrix (right). For
each region in the set of brain regions, the connection strength with every
other region in is represented using different colour intensities. Here,
self-connections are put to zero (represented by white elements in the image).
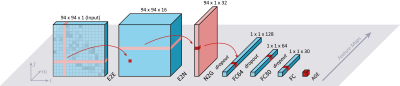
Figure 2: Schematic representation of the network. Image is based on Kawahara et al. The first layer is the edge-to-edge (E2E) convolution layer that sums the edges of node i and j. The second layer is an edge-to-node (E2N), which consists of a 1D convolution filter, and the third convolution layer is a node-to-graph (N2G) layer, which again consists of a 1D convolution filter. The final layers of the network are fully connected (FC) layers. The final output of the network is the prediction of brain age.
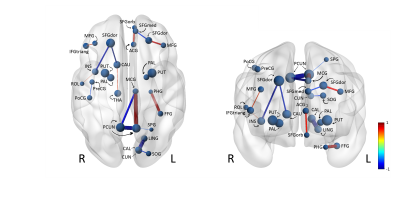
Figure 3: Attribution maps resulting from explainable deep learning analysis from an axial (left) and coronal (right) point of view. The blue edges represent connections that have a negative influence on the prediction score. Whereas the red edges represent connections that positively influence the prediction score. The thickness of the edges corresponds to the absolute value of the attribution.
DOI: https://doi.org/10.58530/2022/0402