0398
Evaluating the capabilities and challenges of layer-fMRI VASO at 3T1FPN, Uni Maastricht, Maastricht, Netherlands, 2Department of Psychiatry, Psychotherapy and Psychosomatics, Christian‐Doppler Medical Centre, PMU, Salzburg, Austria, 3German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 4Department of Radiology, University of Minnesota, Center for Magnetic Resonance Research, Minneapolis, MN, United States, 5Norwegian Centre for Mental Disorders Research (NORMENT), Institute of Clinical Medicine, Uni Oslo, Oslo, Norway, 6Neuroscience Institute, Christian Doppler Medical Centre, PMU, Salzburg, Austria, 7Centre for Cognitive Neuroscience & Department of Psychology, University of Salzburg, Salzburg, Austria
Synopsis
Sub-millimeter functional imaging at ultra high field (UHF) strengths can capture cortical layer-specific functional information flow within and across brain systems. However, UHF scanners are only available to appr. 100 privileged imaging centers, globally. In this work, we implement, characterise and apply a CBV-sensitive VASO sequence setup for widely accessible 3T scanners. We find that the longer T2*, and shorter T1 relaxation times at 3T can account for some of the lower z-magnetization in the inversion-recovery VASO sequence compared to 7T and 9.4T. We conclude that layer-fMRI VASO is applicable without too much further ado at 3T.
Purpose
Recent methodological advances in fMRI contrast and readout strategies allow researchers to approach the mesoscopic regime of cortical layers. This enables the mapping of cortical information processing within and across brain systems. However, most layer-fMRI studies have been confined to specialized ultra-high field (UHF) equipment (7-9.4 Tesla) and venous-biased sequences, which is problematic (Bollmann 2020, Weldon 2020):- The need for UHF scanners limits layer-fMRI applications to <100 MRI labs globally and prohibits the widespread adoption of layer-fMRI as a neuroscientific imaging tool. Layer-fMRI at 3T (Markuerikiaga 2017, Sheeringa 2016, Knudsen 2021) can increase the availability of layer-fMRI worldwide by two orders of magnitude.
- The conventional fMRI contrast of gradient-echo (GE) BOLD imposes unwanted signal bias in the superficial layers. Blood volume sensitive VASO methods can avoid such biases (Lu 2003).
Methods
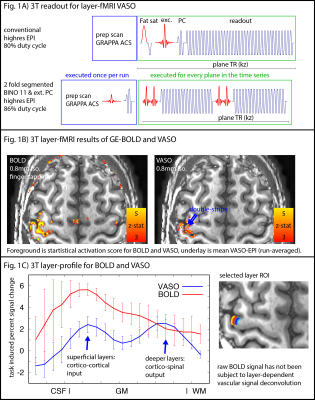
N=12 participants were scanned one a SIEMENS Prisma (64ch coil). We used a 3D-EPI sequence from Stirnberg and Stöcker (2020) for VASO imaging with whole-brain MAGEC capabilities (Huber 2020). Here we used the flexibility of this sequence to optimize layer-dependent CBV acquisitions at 3T:- The T2*-independence of the VASO contrast allows in-plane segmented approaches with increased VASO CNR and reduced BOLD contaminations (Fig 1A). This is necessary, because the conventional BOLD correction methods in VASO of dynamic division of signals can be less effective at long TEs at 3T compared to 7T (Genois 2021).
- The shorter T1 at 3T compared to 7T, allows more aggressive (larger) flip-angles across kz-segments, increasing the readout SNR efficiency.
- The reduced off-resonance artifacts at 3T allow for a larger matrix size and longer echo-train lengths with minimal distortions, ghosting, and typical sub-millimeter EPI phase interference artifacts.
Layer analyses were done in LayNii (Huber et al., 2021).
The visual/motor task consisted of 12 min alternating activation-rest blocks. A movie watching task (Finn 2020) was used for connectivity analyses (HCP clips).
All scan protocols, data and analyses of this study are available for download on Openneuro and Github (https://github.com/layerfMRI/Sequence_Github -> 3T_layerVASO).
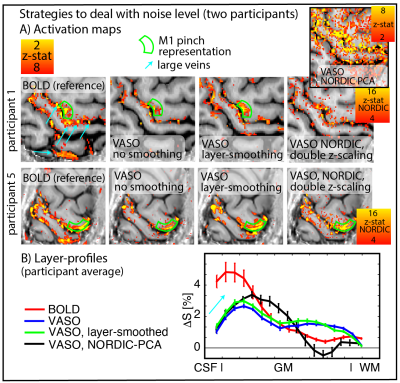
To account for the relatively lower expected functional sensitivity at 3T compared to conventional layer-fMRI field strengths, we investigated the effect of post-processing-based denoising tools. Including anatomically-informed layer-specific spatial smoothing and NORDIC-PCA denoising (Vizioli 2021). We used an implementation of Nordic that requires only magnitude data and work on image rather than raw data space (https://github.com/SteenMoeller/NORDIC_Raw).
Results and Discussion
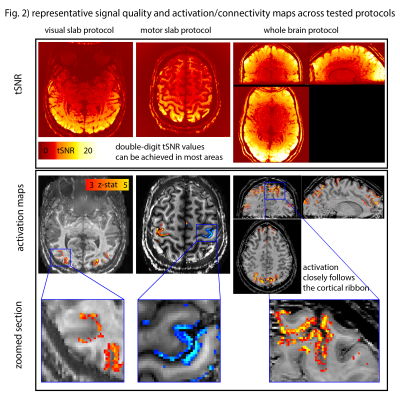
Fig. 1B depicts a single run for BOLD and VASO. The GE-BOLD activation map shows more significantly activated voxels than VASO. VASO’s higher spatial specificity is also visible in profile plots as two separate peaks. The blurry nature of the GE-BOLD signal is expected due to the locally unspecific (intra-vascular) signal at 3T.Fig. 2 illustrates representative results for the different tested protocols. Across all acquisition setups, we found significant activity changes. Due to the locally specificity of CBV-fMRI, the activation pattern closely follows the cortical ribbon.
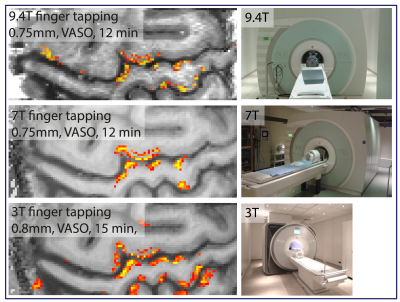
In order to compare the data quality across typical experimental layer-fMRI setups of different field strengths. Two participants were asked to repeat the same finger-tapping task at 3T (32ch coil), at 7T (32ch coil), and at 9.4T (31ch coil). Fig. 3 shows thresholded activation maps of these field strength comparisons. These results suggest surprisingly similar detection sensitivity and very similar layer patterns across field strengths.
Fig. 4 shows activation maps and layer profiles of potential denoising of the acquired data for two representative cases. Layer-smoothing increases the detection sensitivity.
Summary and Conclusion
In this work we find that high quality layer-specific CBV-fMRI data can be acquired on widely available clinical 3T scanners. While the functional detection threshold is limited, we show that layer-dependent activation can be visualized in reasonable scan times of 12 min (primary brain areas) to 45 min (associative brain areas).Due to the vast availability of 3T compared to UHF scanners, we believe that this demonstration of 3T optimized VASO development can boost the user base of laminar fMRI, and helps pave the way for CBV layer-fMRI to become a routine tool to address both clinical and basic neuroscience research questions.
Acknowledgements
We thank Emily Finn for guidance regarding the application of naturalistic tasks.
We thank Luca Vizioli for advice regarding the application of NORDIC-PCA.
Martin Kronbichler was funded by the Austrian Science Fund (FWF grant number: P 30390-B27) and the Scientific Funds of the Paracelsus Medical University (grant number: E-13/18/097-KRO).
Renzo Huber was funded by the NWO VENI project 016.Veni.198.032 and the Maastricht York Partnership (PI: Aneurin Kennerley and Renzo Huber).
Benedikt Poser is partially funded by the NWO VIDI grant 16.Vidi.178.052,by the National Institutes for Health grant R01MH/111444 (PI David Feinberg), and by the H2020 FET-Open AROMA grant agreement no. 88587.
We thank SIEMENS Healthineers for support with sequence code sharing via C2Ps.
We thanks Steen Moeller for his advise on the usage of MR-phase data for NORDIC in our setup.
We thank Scannexus for placing their Prisma to our disposal for phantom sequence testing of the protocols used here.
Data were also provided in part by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University.
References
- Bollmann & Barth (2020), Progr. NeuroBiol., https://doi.org/10.1016/j.pneurobio.2020.101936
- Finn et al., (2019). Nat. Neuro., https://doi.org/10.1038/s41593-019-0487-z
- Huber et al., (2017), Neuron., https://doi.org/10.1016/j.neuron.2017.11.005
- Huber et al., (2020). BioRxiv, doi: https://doi.org/10.1101/2020.06.12.148080
- Knudsen et al., (2021). ISMRM, #2694
- Lu et al. (2003), MRM https://doi.org/10.1002/mrm.10519
- Markuerkiaga et al., (2017), BioRxiv, https://doi.org/10.1101/2020.07.16.206383
- Mueller et al., (2021). ISMRM, https://layerfmri.page.link/ISMR2021
- Scheeringa et al., (2016), PNAS. https://doi.org/10.1016/j.cub.2015.12.038
- Strinberg et al., (2020), MRM, https://doi.org/10.1002/mrm.28486
- Vizioli et al., (2021) Nat Commun. https://doi.org/10.1038/s41467-021-25431-8
- Weldon & Olman, (2020), Philos. Trans. R. Soc. B, https://doi.org/10.1098/rstb.2020.0040
Figures