0397
Sub-0.1 microliter CBV fMRI on the Next Generation 7T scanner1Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States, 3Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 4German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany
Synopsis
Laminar-specific fMRI with CBV-sensitive VASO can address neuroscience research questions on directional information processing within and across brain areas. However, conventional 0.8mm resolutions cannot Nyquist sample the individual layer-groups of interest (e.g. Layers II/III from layer IVc in V1). Here we implement, evaluate, and apply a 0.39-0.45mm (iso.) VASO protocol on the NextGen 7T scanner to resolve layer-features that could not be resolved in humans before. We find that 0.45 mm protocols are possible if the sequence parameters of EPI-segmentation and GRAPPA acceleration are differently optimized than one would do for conventional fMRI protocols.
Purpose
Laminar-specific fMRI with CBV-sensitive VASO is valuable for neuroscience research questions on hierarchical information processing. However, the conventional layer-fMRI resolutions at the order of 0.7-0.8mm do not allow researchers to resolve differential activity of neighboring feedforward and feedback layers that are only 0.5-0.6 mm apart. To resolve, e.g., feedback layers II\III and feedforward layers IVc in the primary visual cortex as two separate peaks, 0.375-0.5 mm resolutions are necessary (Zappe 2008, Goense 2012).The purpose of this study is to implement, conduct, and characterise blood volume sensitive fMRI at 0.45 mm isotropic resolutions with the newly developed hardware of the next generation 7T scanner (Feinberg 2021). This scanner was designed for the purpose of high-resolution fMRI, diffusion and structural imaging of the human neocortex (MRI Corticography).
Methods
HardwareEight, two-hour sessions were conducted on the next generation 7T designed and built under the NIH BRAIN Initiative. The scanner is equipped with a PNS-optimized (Davids 2021) asymmetric head gradient coil (Feinberg 2021), “Impulse” (Siemens Healthineers, Erlangen, Germany). This gradient and scanner are also known under the names “Athena”, “Feinbergatron” or “Corticography” and has a Gmax of 200mT/m (53 mT/m used in the EPI here) and a Smax of 900T/m/s (900T/m/s used for all modules of the readout). We used a 16-channel transmit, 96-channel receive RF coil (Gunamony 2021) with 128 channel receiver system. These higher performance hardware systems of the NexGen 7T scanner enable achieving higher resolution and SNR, reduced distortions and lower g-factor noise than is possible on existing worldwide installed 7T scanners with Gmax of 80mT/m, Smax of 200T/m/s and 32-channel receiver array coils (Beckett 2022).
Sequence
We used a segmented IR 3D-EPI (Stirnberg 2020) as a VASO sequence (Huber 2020). The sequence protocol that was used for functional data acquisitions consisted of 18 partitions, GRAPPA 1-3 (with 5000 fold regularization), TE 19-23ms, PF 6/8 with POCS reconstruction with 8 iterations, in-plane matrix 374-462, 2-6 segments across kz planes, slice thickness 0.39-0.45mm, and in-plane resolution 0.39-0.45mm. The scan protocol pdfs are available on Github: (https://github.com/layerfMRI/Sequence_Github/blob/master/Terra_protocolls). We are happy to share the sequences we used via SIEMENS C2P.
The resolutions used here refer to 0.0911 microliter and 0.0593 microliter volumes, respectively, for 0.45 and 0.39 mm3 isotropic voxels.
Task
Three, 15 min runs of flickering checkerboard stimuli were acquired using a 7T-compatible video projector with extra long throw lens (VPixx Technologies, Inc). PsychoPy scripts are here: https://github.com/layerfMRI/Phychopy_git/tree/master/TappingWithTrTiming. Representative example (raw) data of one session are available here: https://openneuro.org/datasets/ds003850
Data processing
Motion corrected (SPM) data were sorted by CBV and oxygen level contrast and corrected for BOLD contamination (LayNii). Layerification was performed with LN2_LAYERS (Huber 2021) in a small patch of the calcarine sulcus (equi-dist). Block design activation Z-scores were quantified with AFNI and are presented as an overlay to the inherently T1-weighted, time-averaged VASO images.
Results and Discussion
Figures 1, 2 and 3 summarize our main findings during the first (N=6) two-hour scan sessions. Namely, we found that in the thermal-noise dominated regime the protocols need to be differently optimized compared to conventional layer-fMRI VASO. E.g.:- For long readout durations of large matrix sizes, it is advantageous to acquire 3D k-space data across multiple IR-cycles.
- In the thermal noise dominated regime of this resolution it is favourable to use segmentation over GRAPPA within the TR requirements of the task (Guoxiang 2021).
Fig. 5 illustrates the benefit of the sub 0.1 microliter voxel volumes by means of downsampling the data to conventional layer-fMRI resolutions up to 0.9 mm voxels.
In agreement with previous work (Bollermen 2019, Berman 2020, Vizioli 2021), we find that segmented sequence approaches can be advantageous for fMRI resolution acquisitions smaller than 0.7mm.
Summary and Conclusion
In this study we implemented, optimized and conducted 0.39-0.45 mm isotropic VASO imaging on the Next Generation 7T scanner to differentiate layer specific activation in V1 of the human visual cortex that could not be resolved in humans before. We found that even with the advanced hardware of 96ch RF-coils and fast gradients, in the thermal noise dominated regime, it is advantageous to use segmentation as the task timing allows. We found that the isotropic resolution of 0.39-0.45mm reveals layer-dependent signal features that cannot be (Nyquist) separated with conventional 0.8mm layer-fMRI protocols. This achieved higher resolution will allow layer fMRI studies to be performed in thin cortical brain areas as in V1, normally 1.7-1.9mm thick in humans. Future experiments will investigate task-dependent modulations of the individual layer-responses within the primary visual system and beyond. Dissemination of NexGen 7T technology is planned and will provide a novel tool to reach higher spatial resolution in human fMRI research.Acknowledgements
Funding: This project is supported by the NIH BRAIN Initiative (R01MH111444, U01EB025162), 1R44MH129278. Laurentius Huber was funded from the NWO VENI project 016.Veni.198.032.References
- Beckett A, Vu AT, Ahn S, Torrisi S, Polimeni J, Setsompop K, Bilgic B, Stockman J, Feinberg DA. Evaluation of Sub-millimeter resolution in Single-shot EPI on the Next-Generation 7T brain scanner. ISMRM 2022
- Berman AJL, Grissom WA, Witzel T, et al. Ultra-high spatial resolution BOLD fMRI in humans using combined segmented-accelerated VFA-FLEET with a recursive RF pulse design. Magn Res Med. 2020. https://doi.org/10.1002/mrm.28415
- Bollerman S, Staeb D, Barth M. BOLD fMRI with 0.5 mm isotropic voxel size and minimal in-plane distortion using 3D planes-on-a-paddlewheel (POP) EPI at 7 Tesla, ISMRM 2019, 0708. http://indexsmart.mirasmart.com/ISMRM2018/PDFfiles/0708.html
- Davids M, Dietz P, Ruyters G, Roesler M, Klein V, Guerin B, Feinberg DA, Wald L. PNS optimization of a high-performance asymmetric gradient coil for head imaging. ISMRM, #0565, 2021.
- Ding SL, Royall JJ, Sunkin SM, et al. Comprehensive cellular-resolution atlas of the adult human brain. J Comp Neurol. 2016;524:3127-3481. doi:10.1002/cne.24080
- Feinberg DA, Dietz P, Liu C, Setsompop K Mukherjee P Wald L, Vu A, Beckett A Insua I, Schröder M, Stocker S, Bell Pl, Rummert E, Davids M Design and Development of a Next-Generation 7T human brain scanner with high-performance gradient coil and dense RF arrays. In: ISMRM, #0562, 2021.
- Goense JBM, Merkle H, Logothetis NK. High-Resolution fMRI Reveals Laminar Differences in Neurovascular Coupling between Positive and Negative BOLD Responses. Neuron. 2012;76(3):629-639. doi:10.1016/j.neuron.2012.09.019
- Gunamony Müller R, McElhinney P, Williams S, Gross-Weege N, Weiskopf N, Möller H, Feinberg DA A 16-channel transmit 96-channel receive head coil for NexGen 7T scanner. ISMRM #0182, 2021.
- Guoxiang L, Adnan S, Takashi U et al., Comparison of BOLD and VASO in submillimeter high-resolution fMRI Based on BISEPI at 7T, OHBM 2021 #1991.
- Huber L, Finn ES, Chai Y, et al. Layer-dependent functional connectivity methods. Prog Neurobiol. 2020:in print. doi:j.pneurobio.2020.101835
- Huber L (Renzo), Poser BA, Bandettini PA, et al. LAYNII: A software suite for layer-fMRI. Neuroimage. 2021;237:118091. doi:10.1016/j.neuroimage.2021.118091
- Stirnberg R, Stöcker T. Segmented K-Space Blipped-Controlled Aliasing in Parallel Imaging (Skipped-CAIPI) for High Spatiotemporal Resolution Echo Planar Imaging. Magn Reson Med. 2020;85(0):1540-1551. doi:10.1101/2020.06.08.140699
- Vizioli L, Dowdle LT, Moeller S, Yacoub E, and Ugurbil K. New Frontiers of Human Neuroscience: 0.5 mm isotropic 7T in Vivo Human fMRI, lSMRM 2021, https://submissions2.mirasmart.com/ISMRM2021/ViewSubmission.aspx?sbmID=5438&validate=false
- Zappe AC, Pfeuffer J, Merkle H, Logothetis NK, Goense JBM. The effect of labeling parameters on perfusion-based fMRI in nonhuman primates. J Cereb Blood Flow Metab. 2008;28(3):640-652. doi:10.1038/sj.jcbfm.9600564
Figures
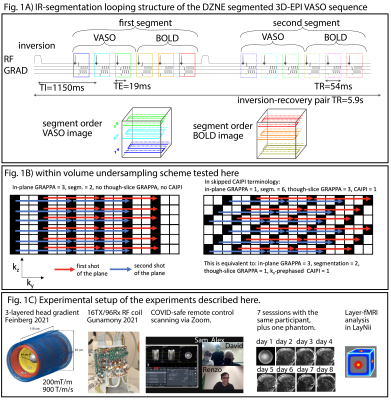
Fig1: Inversion-recovery acquisition of 3D-EPI VASO sequence.
This sequence diagram in panel A) schematically depicts how blood-nulled VASO and control BOLD images were collected across multiple inversion recovery cycles. Panel B) depicts the trajectory of six fold segmented readouts compared to an in-plane accelerated trajectory. Panel C) shows novel hardware of NexGen 7T, the remote data acquisition and layer analysis.
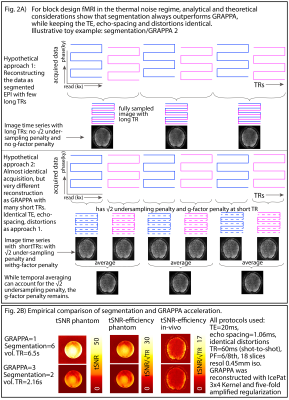
Fig 2: Theoretical and empirical considerations of multi-shot in-plane segmentation for sub-millimeter fMRI
Panels A) and B) depicts theoretically and empirically that in the experimental setup of thermal dominated signal and long block-design tasks, it is advantageous to refrain from (aggressive) GRAPPA acceleration. This is different compared to conventional fMRI.
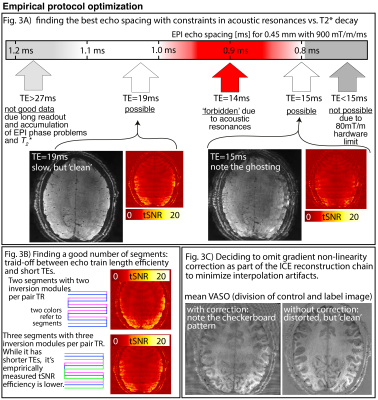
Fig 3: Empirical sequence protocol optimisation in the prototype setup of the Next Generation Hardware.
Panel A) Depicts that we acquired the best data by generously avoiding acoustic resonances (not yet pushing the gradients to their limits). Panel B) depicts that 2 segments are enough to benefit from the shorter echo-train lengths.Panel C) depicts that the data quality was best without gradient non-linearity corrections.
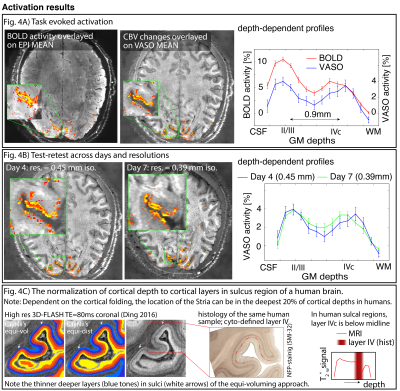
Fig 4: Representative activation results.
It can be seen that the developed acquisition protocol can capture layer-dependent CBV changes at isotropic resolutions of 0.39-0.45mm.
Panel A) As expected from previous (animal) literature, GR-BOLD is more weighted towards the superficial layers due to greater venous contribution than CBV-based fMRI. Panel B) Results are consistent across resolutions and days. Panel C) depicts the expected location of layer IVc dependent on curvature, shown in MRI and histology of human brain (Ding 2016).
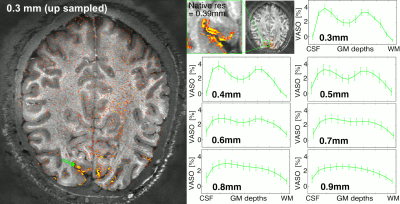
Fig 5: Necessity of sub-0.1 microliter voxels size to capture double stripe (click on GIF to start animation)
The native nominal resolution of 0.39mm was resampled to 0.3-0.9mm resolutions. And the layer profile was extracted from the same chunk of the calcarine sulcus.
It is visible that with larger voxel sizes, progressively more layer-groups are becoming part of the same voxels (partial voluming).