0393
Corpus callosum lesions are associated with worse cognitive performance in cerebral amyloid angiopathy1Leiden University Medical Center, Leiden, Netherlands, 2Massachusetts General Hospital, Boston, MA, United States, 3Ribeirão Preto medical School, São Paulo, Brazil, 4University Medical Center Utrecht, Utrecht, Netherlands
Synopsis
We assessed the contribution of small vessel disease lesions in the corpus callosum (CC) to vascular cognitive impairment in cerebral amyloid angiopathy (CAA). A total number of 21 CC lesions was found in 19/65 (29%) CAA patients. CC lesion presence was associated with reduced microstructural white matter integrity within the CC and in the whole brain white matter. Patients with CC lesions performed significantly worse on multiple cognitive domains compared to those without CC lesions after correcting for relevant covariates. Together, our findings suggest that CC lesions independently contribute to cognitive impairment through strategic microstructural disruption of white matter tracts.
Background
Cerebral amyloid angiopathy (CAA) is highly prevalent in Alzheimer’s disease (AD) and is associated with hemorrhagic and ischemic brain lesions [1]. CAA is increasingly recognized as an important cause of cognitive impairment, independent of the severity of classical AD pathology or the presence of large intracerebral hemorrhage (ICH). However, the mechanisms underlying cognitive impairment in these patients are poorly understood. We hypothesized that lesions in the corpus callosum (CC), because of their strategic location [2], are associated with widespread reductions in microstructural white matter integrity and worse cognitive performance, specifically in the domains of attention/information processing speed and executive functioning.Methods
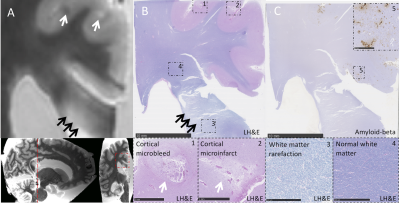
65 patients with probable CAA underwent 3T MRI, including a diffusion tensor imaging scan (TR, 8000ms; TE, 82ms; voxel size, 2.0mm3 isotropic; 64 gradient directions with a b-value of 700 s/mm2 and one b=0 s/mm2 image), and neuropsychological testing. Microstructural white matter integrity was quantified by fractional anisotropy (FA) and mean diffusivity (MD) [3, 4]. The average FA and MD of the CC (not excluding any lesions) and the rest of the white matter (excluding the CC and corticospinal tract because the brainstem was not included in the field of view for all participants) were computed for each participant [4]. In patients with unilateral ICH (n=31), we excluded the diffusion metrics in the affected hemisphere to account for the possible effects of ICH on the microstructural white matter integrity. FLAIR white matter hyperintensity (WMH) volume was calculated using an automated method similar to the one previously described [5, 6]. In patients with unilateral ICH (n=31), we excluded the WMH volume in the affected hemisphere, and multiplied the WMH volume in the unaffected hemisphere by two to account for the possible effects of ICH on WMH volume. Normalized WMH was expressed as the percentage of estimated intracranial volume (ICV) from the FreeSurfer parcellation. Z-scores on individual neuropsychological tests were averaged into five cognitive domains: information processing speed, executive functioning, memory, language, and visuospatial ability. CC lesions were defined as hemorrhagic (microbleeds or larger bleeds) or ischemic (microinfarcts, larger infarcts, diffuse fluid-attenuated inversion recovery hyperintensities) (figure 1). Associations between CC lesion presence, microstructural white matter integrity, and cognitive performance were examined with multiple regression models. The prevalence of CC lesions was confirmed in an independent validation cohort of memory clinic patients with and without CAA (n=82). In parallel, we assessed CC lesions on ex vivo MRI in CAA patients (n=19) and controls (n=5) and determined associated tissue abnormalities with histopathology.Results
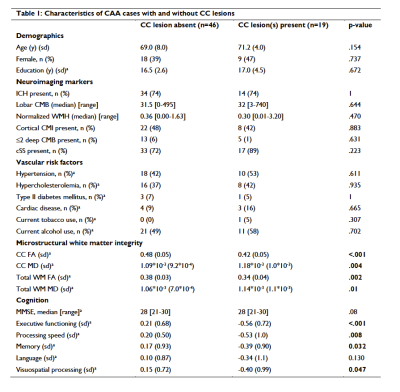
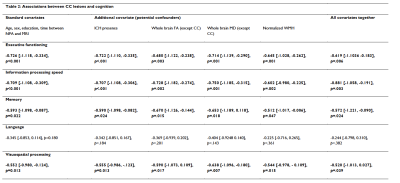
A total number of 21 CC lesions was found in 19/65 (29%) CAA patients. Baseline characteristics of patients with and without CC lesions are described in table 1. After correcting for age, sex, and normalized WMH volume, CC lesion presence was significantly associated with decreased microstructural white matter integrity within the CC (standardized beta coefficient [95% CI] FA = -0.467 [-0.685, -0.248], p <0.001; MD = 0.343 [0.115, 0.572], p = 0.005) and in the whole brain white matter (excluding the CC) (standardized beta coefficient [95% CI] FA = -0.414 [-0.634, -0.194], p < 0.001); MD = 0.337 [0.111, 0.562], p = 0.005). Patients with CC lesions performed significantly worse on all cognitive domains except language, compared to those without CC lesions after correcting for relevant covariates, including age, sex, education, time between neuropsychological assessment and MRI, presence of large ICH, whole brain FA and MD (excluding the CC), and normalized white matter hyperintensity volume (table 2). The results did not change when patients with bilateral ICH were excluded from the analyses. In the validation cohort, CC lesions were present in 14/54 (26%) patients with probable and 2/8 (25%) patients with possible CAA, and in 3/20 (15%) patients without CAA. In the ex vivo cohort, CC lesions were present in 10/19 (53%) patients and 2/5 (40%) controls. On histopathology (figure 2), ischemic CC lesions were associated with tissue loss and demyelination, which extended beyond the lesion core.Discussion
Together, these data suggest that CC lesions are a frequent finding in cerebral amyloid angiopathy, and that they independently contribute to cognitive impairment through strategic microstructural disruption of white matter tracts.Acknowledgements
The authors would like to acknowledge funding support from the National Institutes of Health (RF1 NS110054 to B.J.B., R00 AG059893 to S.J.v.V., and R01 AG026484 to S.M.G.) and the Netherlands Organization for Scientific Research (Veni 91619021 to S.J.v.V.).References
[1] Charidimou A, Martinez-Ramirez S, Reijmer YD, et al. Total Magnetic Resonance Imaging Burden of Small Vessel Disease in Cerebral Amyloid Angiopathy: An Imaging-Pathologic Study of Concept Validation. JAMA neurology. Aug 1 2016;73(8):994-1001. doi:10.1001/jamaneurol.2016.0832
[2] Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clinical science (London, England : 1979). Apr 25 2017;131(8):715-728. doi:10.1042/cs20160452
[3] Leemans A, Jones DK. The B-matrix must be rotated when correcting for subject motion in DTI data. Magnetic resonance in medicine. Jun 2009;61(6):1336-49. doi:10.1002/mrm.21890.
[4] Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. Apr 1 2008;40(2):570-582. doi:10.1016/j.neuroimage.2007.12.035
[5] Hedden T, Van Dijk KR, Shire EH, Sperling RA, Johnson KA, Buckner RL. Failure to modulate attentional control in advanced aging linked to white matter pathology. Cerebral cortex (New York, NY : 1991). May 2012;22(5):1038-51. doi:10.1093/cercor/bhr172.
[6] Xiong L, Boulouis G, Charidimou A, et al. Dementia incidence and predictors in cerebral amyloid angiopathy patients without intracerebral hemorrhage. J Cereb Blood Flow Metab. Feb 2018;38(2):241-249. doi:10.1177/0271678x17700435
Figures
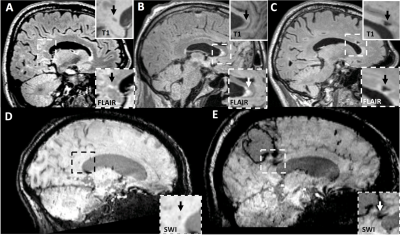
Figure 1: Representative examples of corpus callosum lesions. (A) Cerebral microinfarct located in the splenium of the corpus callosum. (B) Diffuse FLAIR hyperintensity located in the genu of the corpus callosum. (C) Lacunar infarct located in the genu of the corpus callosum. (D) Cerebral microbleed located in the splenium of the corpus callosum. (E) Extension of intracerebral hemorrhage into the splenium of the corpus callosum.