0384
Combined 23Na and pTx-1H Imaging for Renal MRI at 7 Tesla1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander- Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Faculty of Electrical Engineering and Information Technology, University of Applied Sciences, Aachen, Germany, 3Rapid Biomedical GmbH, Rimpar, Germany, 4Siemens Healthcare GmbH, Erlangen, Germany, 5Department of Neuroradiology, University Hospital Erlangen, Friedrich-Alexander- Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 6Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
In the human kidneys, the cortico-medullary sodium gradient plays an important role as it is essential for the concentration of urine and changes may indicate renal malfunction. However, quantitative 23Na MRI requires high-quality 1H MRI, since this facilitates co-registration and correction of partial volume effects, which is needed for body imaging in particular. We therefore show the feasibility of high-quality renal proton and sodium imaging using an 8Tx/16Rx 1H transceiver array that can be combined with a 23Na four-rung birdcage coil in a single setup.
Introduction
In the human kidneys, the cortico-medullary sodium (23Na) gradient plays an important role as it is essential for the concentration of urine and changes may indicate renal malfunction.1 Due to its limited signal-to-noise ratio (SNR) 23Na MRI is challenging at low field strengths. Therefore, 23Na imaging particularly benefits from ultra-high field (UHF) strength, since SNR increases at least linearly with field strength.2 Nevertheless, for quantification, high-quality proton (1H) images are crucial, e.g. to correct for partial volume effects3. This applies in particular for body imaging, where the resolution of 23Na MRI has to be decreased in comparison to head imaging and partial volume effects enlarge. However, 23Na body coils for UHF are rare and usually have no 1H channel or only single channel transmission.4,5 Consequently, additional 1H images are acquired at lower field strengths like 1.5T or 3T, which leads to longer scan times and complicates image co-registration when patients have to be repositioned. On the other hand, at 7T, the shorter Larmor wavelength is challenging for 1H imaging in the body, but the acquisitions highly benefit from parallel transmission (pTx) techniques.2,6 Hence, we show the feasibility of high-quality renal 1H and 23Na imaging using an 8Tx/16Rx 1H transceiver array combined with a 23Na four-rung birdcage in a single setup.Methods
One volunteer was scanned in accordance with the institutional guidelines and with approval of the local ethics committee on a 7T MR system (MAGNETOM Terra, Siemens Healthcare GmbH, Erlangen, Germany) using an 8Tx/16Rx transceiver array combined with a 1Tx/1Rx 23Na four-rung birdcage (Rapid Biomedical GmbH, Rimpar, Germany) shown in Figure 1. The 1H MRI was performed in pTx mode of the scanner with local/global SAR limits in first level mode (IEC60601-2-33) met by limiting the radio-frequency (RF) power of each transmit channel. For 23Na imaging, the scanner had to be switched to 1Tx mode: switching takes about 6min and could be performed with the subject remaining within the scanner.Proton imaging:
T1- and T2*-weighted 1H MRI measurements (see Table 1) were performed with three different B1+-shim sets: 1. phase of all Tx-channels equal zero (“Null Phase Shim”), 2. vendor provided B1+-shim for heart imaging7 (“Heart Shim”), 3. an individual hybrid B1+-shim trading homogeneity and transmit efficiency. For the latter, relative B1+-maps8 were acquired and the shim values for every Tx-coil c were calculated by minimizing the function $$$ cost = 0.8\cdot CoV^2 + 0.2 \cdot \eta^{-2} $$$, with $$$ CoV = \frac{std\left( \left| \sum^C_{c=1} B_{1,c}^+ (r) \right| \right)}{mean\left( \left| \sum^C_{c=1} B_{1,c}^+ (r) \right| \right)} $$$, the coefficient of variation, and the mean efficiency $$$ \eta = mean \left( \frac{\left| \sum^C_{c=1} B_{1,c}^+ (r) \right| }{ \sum^C_{c=1}\left| B_{1,c}^+ (r) \right| }\right) $$$ in a region of interest around the kidneys. All 1H images were acquired under breath-hold in exhaled state.
Sodium imaging:
The 23Na measurements were carried out using a density-adapted radial sequence9 (parameters shown in Table 1) under free-breathing. The images were reconstructed with and without retrospective self-gating for respiration5 using a NUFFT-based gridding10. The signal was then normalized to a bottle containing 50mmol/L NaCl solution. Moreover, SNR was calculated in the whole kidney.
After the measurements, the hybrid B1+-shimmed 1H images were co-registered to the 23Na images via a non-rigid registration.11
Results
Figure 2 shows the B1+-maps of the different shims. With the Null Phase Shim the coefficient of variation COVnull = 0.37 and the efficiency ηnull = 0.5, the Heart Shim showed an equal COVheart = 0.38 but less efficiency ηheart = 0.46, whereas the hybrid B1+-shim had better homogeneity COVhybrid = 0.3 and was much more efficient ηhybrid = 0.7. In Figure 3, the measurements of the different 1H GRE contrasts are presented. While the Null Phase Shim had signal dropouts, the Heart Shim showed overall poor contrast. The hybrid B1+-shim offered good contrast without any signal dropouts. The volunteer had a cyst in the right kidney, which could be best identified in the individual B1+-shimmed acquisitions. Figure 4a shows the 23Na acquisition with a SNR = 19.5 in the whole kidney. In Figure 4b, the 23Na image is overlaid with the co-registered T1-weighted 1H contrast. The co-registration still showed some imperfections: without self-gating, the left kidney is shifted in head-feet direction and with self-gating (exhaled state), the right kidney was slightly displaced.Discussion and Conclusion
In this work, we demonstrated the feasibility of combined high-quality 1H and 23Na imaging of human kidneys at 7T. No B0, B1 and partial volume corrections of the 23Na images have been performed so far. Thus, no 23Na concentrations for the medulla and cortex of the kidneys and neither the cortico-medullary gradient, have been specified yet.Overall, the proposed setup increases patient comfort and reduces scanner time compared to previously used approaches for UHF, as both 1H and 23Na MRI can be performed without re-positioning of the patient. Moreover, it facilitates image co-registration and offers the possibility of interleaved 1H/23Na measurements12 in the human body. Due to its large coverage, this setup can further be used for combined 1H/23Na acquisitions in several other body regions, e.g. the heart, liver or intervertebral disks.
Acknowledgements
No acknowledgement found.References
1. Zollner FG, Konstandin S, Lommen J, Budjan J, Schoenberg SO, Schad LR, et al. Quantitative sodium MRI of kidney. NMR Biomed. 2016;29(2):197-205.
2. Mark E. Ladd PB, Martin Meyerspeer, Ewald Moser, Armin M. Nagel, David G. Norris, Sebastian Schmitter, Oliver Speck, Sina Straub, Moritz Zais. Pros and cons of ultra-high-field MRI/MRS for human application. Progress in Nuclear Magnetic Resonance Spectroscopy. 2018;109:1-50.
3. Niesporek SC, Hoffmann SH, Berger MC, Benkhedah N, Kujawa A, Bachert P, et al. Partial volume correction for in vivo (23)Na-MRI data of the human brain. NeuroImage. 2015;112:353-63.
4. Boehmert L, Kuehne A, Waiczies H, Wenz D, Eigentler TW, Funk S, et al. Cardiorenal sodium MRI at 7.0 Tesla using a 4/4 channel (1) H/(23) Na radiofrequency antenna array. Magn Reson Med. 2019;82(6):2343-56.
5. Platt T, Umathum R, Fiedler TM, Nagel AM, Bitz AK, Maier F, et al. In vivo self-gated (23) Na MRI at 7 T using an oval-shaped body resonator. Magn Reson Med. 2018;80(3):1005-19.
6. de Boer A, Hoogduin JM, Blankestijn PJ, Li X, Luijten PR, Metzger GJ, et al. 7 T renal MRI: challenges and promises. Magma. 2016;29(3):417-33.
7. Reiter T, Lohr D, Hock M, Ankenbrand MJ, Stefanescu MR, Kosmala A, et al. On the way to routine cardiac MRI at 7 Tesla - a pilot study on consecutive 84 examinations. PloS one. 2021;16(7):e0252797.
8. Van de Moortele PF, Ugurbil K, editors. Very Fast Multi Channel B1 Calibration at High Field in the Small Flip Angle Regime. Proceedings of the 17th ISMRM & SMRT Annual Meeting; 2009; Hawai.
9. Nagel AM, Laun FB, Weber MA, Matthies C, Semmler W, Schad LR. Sodium MRI using a density-adapted 3D radial acquisition technique. Magn Reson Med. 2009;62(6):1565-73.
10. BART. Toolbox for Computational Magnetic Resonance Imaging. 2015.
11. Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. elastix: a toolbox for intensity-based medical image registration. IEEE transactions on medical imaging. 2010;29(1):196-205.
12. de Bruin PW, Koken P, Versluis MJ, Aussenhofer SA, Meulenbelt I, Bornert P, et al. Time-efficient interleaved human (23)Na and (1)H data acquisition at 7 T. NMR Biomed. 2015;28(10):1228-35.
Figures

Table 1: Sequence parameters for the 1H GRE measurements and the 23Na DA-3D-RAD acquisition
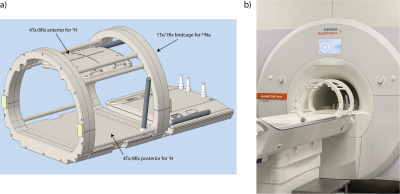
Figure 1: a) Design of the coil setup with a four-rung birdcage for 23Na imaging and two parts for 1H imaging: an anterior and a posterior transceiver array with 8 channels, each. All 16 coil elements are paired to 8Tx elements during transmission to match the 8 available Tx channels of the MR system. b) Coil setup in the MRI-scanner.
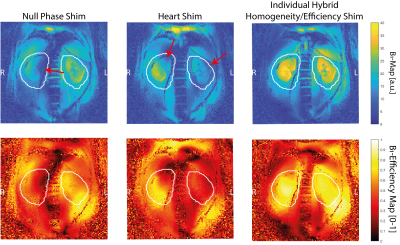
Figure 2: Relative B1+-maps of the different shim sets (upper row) and efficiency maps (lower row): whereas the null phase shim shows signal dropouts in the kidneys, the heart shim performs better, but also has much lower efficiency and homogeneity than the individual hybrid-shim.
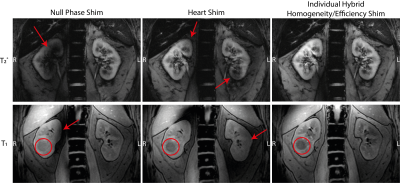
Figure 3: T2* (upper row) and T1 (lower row) 1H acquisitions with the different shims. In the null phase shim, severe signal dropouts are observable especially in the T2* image, while the heart shim shows low contrast in the T1 acquisition and also some shadows in the T2* image. The individual hybrid-shim shows good contrast and no signal dropouts in both weightings. The volunteer has a cyst in his right kidney (red circle; only observable in the T1 images due to a different slice selection), which can be best identified in the hybrid-shimmed images.
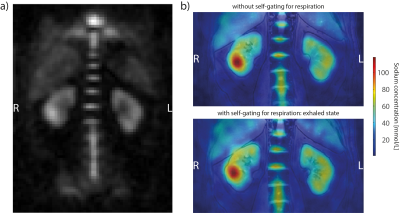
Figure 4: a) 23Na MRI of the kidneys without intrinsic respiratory sorting. b) The 23Na image overlaid over the co-registered 1H image without (upper) and with (lower) respiratory sorting.