0383
First in-vivo 23Na human imaging at 10.5T using a combined 23Na-loop 1H-dipole transceiver array1Center for Magnetic Resonance Research, University of Minnesota, Minneapolis, MN, United States, 2Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
Synopsis
Performing sodium and proton imaging in the same study can provide a wealth of unique multinuclear insights for the study of pathologies and their treatment. However, the coils to perform such studies in the human body are limited and none were validated for human use at 10.5T until now. While both nuclei can benefit from increased SNR at 10.5T sodium suffers from low SNR due to the lower gyromagnetic ratio and natural abundance in the human body compared to 1H. First in-vivo results are presented for a 10.5T sodium-proton torso array following a safety validation study determining safe operating limits.
Introduction
23Na imaging generally suffers from low SNR due to the lower gyromagnetic ratio compared to 1H as well as the lower natural abundance in the human body. Therefore, previously a combined 23Na-1H transceiver array consisting of eight 23Na-loops and eight 1H-dipoles for imaging the body at 10.5T was proposed1 to take advantage of the expected increase in SNR afforded by 10.5T. Here, we demonstrate the validation of the electromagnetic (EM) model of this array and present the first 23Na images acquired at 10.5T in the human body.Methods
MRI experiments were conducted on a whole-body 10.5T scanner (Siemens Healthcare, Erlangen, Germany). The coil array was connected to the scanner via two 8-way splitters with fixed relative phases between the outputs. Separate TR-switches for sodium and proton were used, each optimized for the respective frequencies of 118Mhz and 447MHz.Coil validation
To validate the EM model of the coil, experimental data was acquired in a polyvinylpyrrolidone (PVP)-agar phantom model (c.f. Tab.1) with a cross section approximating a human torso that contained a small container filled with oil to obtain a phase reference for MR thermometry2. All MR experiments were conducted on a whole-body 10.5T magnet.
Relative B1+ maps were acquired for both nuclei on a channel-wise basis3. In addition, absolute B1+ mapping was performed for 23Na on a series of GRE images with varying flip angle, while the AFI4 method was used for 1H. For both nuclei, the absolute B1+ maps were acquired for two different B1+ shims (CP+ and CP2+) by changing the order the elements were connected to the 8-way splitters. Relative and absolute B1+ maps were combined to obtain channel-wise absolute B1+ maps. Based on these, channel-wise real-valued scaling factors were determined to rescale the simulation data.
Using the same two B1+ shims, experimental SAR distributions were acquired based on MRT data. To this end, a multi-echo GRE (TE=5ms/10ms/15ms) acquisition was performed (using the 1H elements) before and after a ten-minute long heating sequence (Pavg,23Na=145W; Pavg,1H=119W). All temperature data was corrected for a DC offset determined in the small oil container. SAR was calculated based on the measured temperature difference as . For comparison, the simulated SAR was averaged over the same voxel volume (4x4x4mm3).
Regions of low B1+ and SAR were excluded when calculating the normalized root mean square error (NRMSE) between experimental and simulation data.
To determine safe operating power limits, EM simulations were performed in the Duke, Ella, and Fats29 model (Sim4Life, Zurich Medtech, Zürich, Switzerland) with the arrays positioned to target the kidneys. Using the fixed relative phases of the 8-way splitters, peak 10g SAR was evaluated for the CP+ shim for both nuclei across all three models. Based on the maximum peak 10g SAR values, total power limits of 71W for 23Na and 30W for 1H were determined.
In-vivo study
Imaging was performed on a healthy male subject targeting the kidneys and spine, following an IRB approved protocol with an FDA Investigational Device Exemption. T1 weighted gradient echo images with fat suppression were acquired using the 1H elements during breath hold. In addition, a 3D radial density adapted sequence5 was used for 23Na imaging. Imaging parameters are summarized in Table 2.
Results
A comparison of the simulated and experimental channel-wise B1+ maps is shown in Figure 1 together with the channel-wise scaling factors: 0.60±0.03 for 23Na and 0.35±0.07 for 1H (Fig.1c). The B1+ maps displayed in Figure 2 correspond to a NRMSE of 0.14/0.17 and 0.39/0.24 for the CP+/CP2+ mode for 23Na and 1H, respectively. A similar comparison of the SAR maps is shown in Figure 3, yielding NRMSE of 0.35/0.35 and 0.28/0.18.In-vivo results of a coronal slice targeting the kidneys, as well as a sagittal slice through the spine are summarized in Figure 4.
Conclusion
In this work we successfully validated the EM model of a combined 23Na-1H transceiver array consisting of eight 23Na-loops and eight 1H-dipoles for imaging the body at 10.5T and subsequently acquired the first in-vivo human 23Na images at 10.5T. While the initial in-vivo study shows promising results, an in-depth analysis is still required to quantify the expected SNR gains at 10.5T as well as potential limitations of 23Na body imaging with regard to total power and SARAcknowledgements
Funding was provided by NIH P41 EB027061References
[1] Erturk, A., Lagore, R.L., Auerbach, E.J., Kobayashi, N., Adriany, G., Ugurbil, K. and Metzger G.J. (2019), Design and implementation of a combined sodium-loop proton-dipole transceiver array for body imaging at 10.5 Tesla. In Proceedings of the 27th Annual Meeting of the ISMRM.
[2] J. C. Hindman (1966), Proton Resonance Shift of Water in the Gas and Liquid States, J. Chem. Phys. 44, 4582-4592. https://doi.org/10.1063/1.1726676
[3] Van de Moortele, P-F and Ugurbil, K. (2009), Very Fast Multi Channel B1 Calibration at High Field in the Small Flip Angle Regime. In Proceedings of the 17th Annual Meeting of the ISMRM.
[4] Yarnykh, V.L. (2007), Actual flip-angle imaging in the pulsed steady state: A method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn. Reson. Med., 57: 192-200. https://doi.org/10.1002/mrm.21120
[5] Nagel, A.M., Laun, F.B., Weber, M.-A., Matthies, C., Semmler, W. and Schad, L.R. (2009), Sodium MRI using a density-adapted 3D radial acquisition technique. Magn. Reson. Med., 62: 1565-1573. https://doi.org/10.1002/mrm.22157
Figures
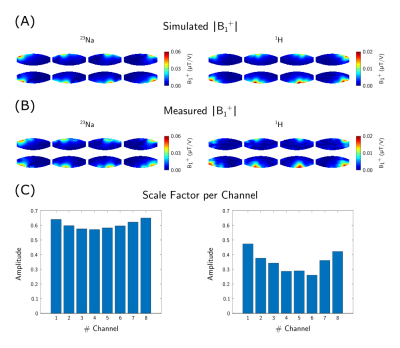
Figure 1:
Channel-wise absolute B1+ maps of the simulation (A) and experimental data (B). The simulation data was scaled by the channel-wise scale factors (C).
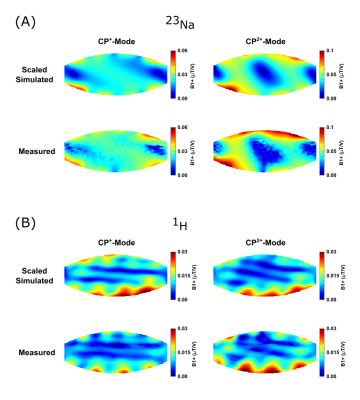
Figure 2:
B1+ comparison between scaled simulation results and experimental data for both
shims and nuclei.
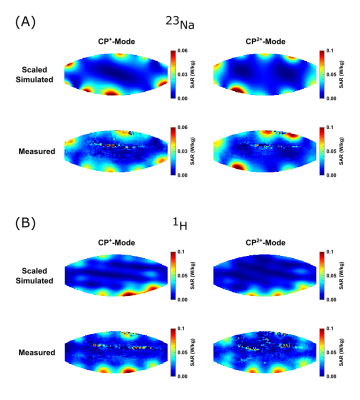
Figure 3:
SAR comparison between scaled simulation results and experimental data for both shims and nuclei.
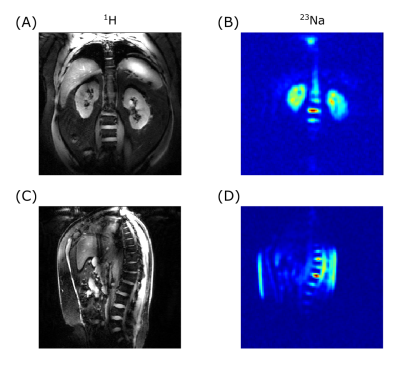
Figure 4:
In-vivo data targeting the kidneys (A+B) and the spine (C+D)
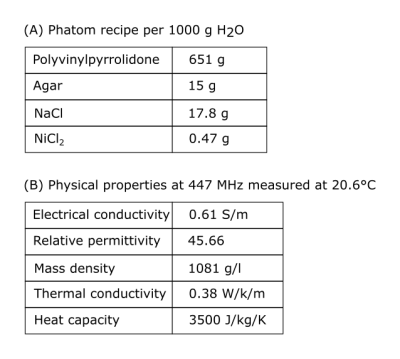
Table 1:
Phantom properties