0379
Magnetic resonance imaging of cationic compounds using their proton exchange modulating effect1Radiology, Johns Hopkins University, Baltimore, MD, United States, 2F.M Kirby Research Center, Kennedy Krieger Institute, Baltimore, MD, United States, 3Biomedical Engineering, Johns Hopkins University, Baltimore, MD, United States, 4Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 5Oncology, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Here we report a novel approach to detect non-labeled cationic compounds, named modulated saturation transfer (MOST)-MRI, based on their effect of modulating the exchange rate of surrounding exchangeable protons. MOST-MRI was able to detect polyethylenimine (PEI600, M.W. = 600 Da), at a high sensitivity (μM or μg/mL range) through its quenching effects on surrounding fast-exchanging protons. In animals, MOST-MRI detected the tumor uptake of PEI600 that was injected intratumorally at a low dose (50 μg/kg b.w.), indicating the potential of MOST-MRI for label-free imaging guidance that is needed for the development and clinical translation of cationic gene delivery systems.
Introduction
Determining the accumulation, elimination, and degradation of cationic gene delivery vectors in targeted tissues is of great importance for monitoring the success of gene therapy to improve therapeutic outcome1. Current tracking methods all employ chemical labelling2-5, but the conjugated imaging labels may alter the pharmacokinetics and pharmacodynamics of the vectors and generate false positives if dissociated in vivo. Herein, we reported a new contrast generating MRI technology, namely modulated saturation transfer (MOST), which indirectly detects cationic vectors in vivo through their modulating effects on the proton exchange rates of surrounding biomolecules, providing an approach for image-guided gene therapy.Methods
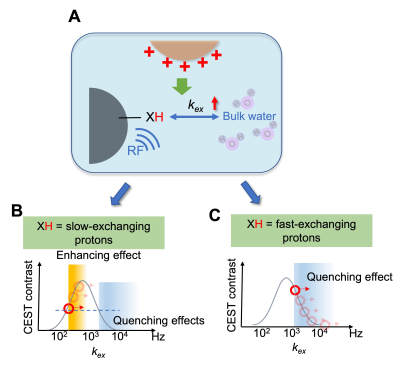
TheoryChemical Exchange Saturation Transfer (CEST) contrast is generated by exchangeable protons with an exchange rate (kex) in the slow-to-intermediate exchange regime (Figure 1A). Other compounds in close environment can affect this exchange rate6, which can be described by:
$$k_{ex}= k_{ex0}+k_{modulating}∙[modulator]$$
where kmodulating represents a modulator’s ability to alter labile proton exchange in a concentration-dependent manner, and kex0 represents the inherent exchange rate. We hypothesized that the presence of a modulator should be detectable in vivo through its effect on endogenous CEST signals. For instance, increasing kex using a modulator would increase CEST contrast in the low exchange rate regime but will decrease CEST contrast if kex becomes larger than the optimal rate determining the exchange regime for a particular field.7-9 Hence, a modulator that can accelerate kex, in principle, will enhance the CEST contrast of slow-exchanging protons till kex reaches the optimal magnitude (orange region, Figure 1B). Enhancement can also be achieved by quenching the CEST contrast of fast-exchanging protons to a detectable exchange regime (blue region, Figure 1C).
In vitro MRI
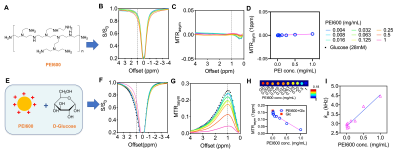
Phantoms consisting of 28 mM D-glucose with 0.004 to 1 mg/mL PEI600, a commonly used non-viral gene delivery vector10,11 were imaged using a 9.4 T vertical bore Bruker MRI scanner at 37 oC using B1 from 1.2 to 5.9 μT. The WASSR method (B1=0.5 uT, Tsat = 500 ms) was used for B0 correction12. Detection sensitivity was defined as the minimal PEI600 concentration needed to generate a contrast to noise ratio (CNR) of 2√2 between the samples containing both D-glucose/PEI600 and D-glucose alone. PBS (pH 7.4) was used as the buffer.
Animals and in vivo MRI
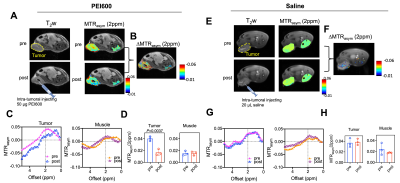
Subcutaneous pancreatic tumors were prepared by inoculating syngeneic KrasLSL.G12D/+; p53R172H/+; PdxCretg/+ (KPC)-derived PDAC cells (2x106 in 200 μL) subcutaneously into the flanks of C57BL/6J mice (n=3). After 2 weeks, in vivo MRI was conducted before and 10 minutes after intratumoral injection of PEI600 (50 μg PEI600 in 20 μL saline). An 11.7 T Bruker Biospec horizontal scanner equipped with mouse brain surface array RF coil (receiver) and 72 mm volume coil (transmitter) was used. A RARE-based T2-weighted sequence was used to acquire anatomical images (TR/TE=2.1 s/17 ms, RARE factor=16, slice thickness=1 mm, slice number=4; matrix size=128×128, field of view=25 mm×25 mm, number of averages=4). Multi-slice CEST images were acquired using the following parameters: B1=2 μT, Tsat=3 s, offsets ranging from −4 to +4 ppm (step=0.2 ppm), RARE factor=23, TR/TE=12.3 s/18.6 ms, number of averages=2, matrix size =64×64, field of view=25 mm×25 mm, number of slice=4. The tumor regions where 20 μL saline was locally injected, and muscle regions were used as the controls.
Results
We characterized the proton-exchange modulating effect of PEI600 on D-glucose, a widely studied CEST agent that is abundant in vivo (~5 mM)13,14. At pH 7.4, PEI600 alone exhibits negligible CEST contrast (Figures 2A-D). When mixed with D-glucose (28 mM), PEI600 quenched the exchange rate, providing an increased CEST signal of D-glucose over a broad frequency range (0-4 ppm) (Figure 2E-H). The MTRasym(1ppm) values were inversely proportional to PEI600 concentrations (Figure 2H), and the modulating effect was still detectable as low as 8 μg/mL (or 13 µM). Then, we calculated the apparent kex of glucose at different concentrations of PEI600 by fitting the multi-B1 Z-spectral data using 2-pool Bloch-McConnell equations (BME)15, which revealed kex increased linearly with PEI600 concentration (Figure 2I).In animals, intratumorally injected PEI600 caused a substantial decrease in MTRasym values at the tumor injection site (Figure 3A-D), where saline injection in the tumor had negligible effects (Figure 3E-H). Comparing the pre- and post-injection MTRasym plots revealed the maximum decrease of endogenous CEST contrast occurred between 2 and 3 ppm (Figure 3C). Quantified by MTRasym (2ppm), the CEST contrast in the tumors decreased from 0.0401 to 0.0169, corresponding to a 57.9% signal change (p=0.0037, Figure 3D), whereas no significant change of CEST contrast was observed in the muscle or saline-injected tumors (Figure 3D).
Discussion and Conclusions
We demonstrated that MOST-MRI provides high sensitivity for detecting cationic compounds that are intrinsically MRI invisible. The technology is based on the exchange-modulating effect on surrounding CEST agents by a modulator such as cationic polymer PEI. MOST-MRI can detect PEI600 in µM or µg/mL range, which is directly applicable to therapeutic applications, given the doses of cationic gene vectors commonly used in preclinical studies are 0.33-3.3 mg/kg PEI (using N/P ratio of 516 for carrying 0.5-5 mg/kg DNA/RNA17-19). In comparison, this dose range is approximately 1000 times less than that of glucose CEST MRI. Hence, MOST-MRI potentially permits label-free theranostic MRI tracking of gene delivery agents.Acknowledgements
This work was supported by R01CA211087 and National Multiple Sclerosis Society (NMSS) PP-1908-34973.References
1. Malek, A. et al. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI(-PEG)/siRNA complexes. Toxicol Appl Pharm 236, 97-108, (2009).
2. Mohammadinejad, R. et al. Shedding light on gene therapy: Carbon dots for the minimally invasive image-guided delivery of plasmids and noncoding RNAs - A review. J Adv Res 18, 81-93, (2019).
3. Li, D. et al. Theranostic nanoparticles based on bioreducible polyethylenimine-coated iron oxide for reduction-responsive gene delivery and magnetic resonance imaging. Int J Nanomed 9, 3347-3361, (2014).
4. Wang, X. Y. et al. In Vivo Tracking of Fluorinated Polypeptide Gene Carriers by Positron Emission Tomography Imaging. Acs Appl Mater Inter 12, 45763-45771, (2020).
5. Hajdu, I. et al. A (89)Zr-labeled lipoplex nanosystem for image-guided gene delivery: design, evaluation of stability and in vivo behavior. Int J Nanomedicine 13, 7801-7818, (2018).
6. Liepinsh, E. & Otting, G. Proton exchange rates from amino acid side chains--implications for image contrast. Magn Reson Med 35, 30-42, (1996).
7. van Zijl, P. C. & Yadav, N. N. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 65, 927-948, (2011).
8. Liu, G., Song, X., Chan, K. W. & McMahon, M. T. Nuts and bolts of chemical exchange saturation transfer MRI. NMR Biomed. 26, 810-828, (2013).
9. Zaiss, M. & Bachert, P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys. Med. Biol. 58, R221-269, (2013).
10. Hao, F. et al. Polyethylenimine-based Formulations for Delivery of Oligonucleotides. Curr Med Chem 26, 2264-2284, (2019).
11. Zakeri, A. et al. Polyethylenimine-based nanocarriers in co-delivery of drug and gene: a developing horizon. Nano Rev Exp 9, 1488497, (2018).
12. Kim, M., Gillen, J., Landman, B. A., Zhou, J. & van Zijl, P. C. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med 61, 1441-1450, (2009).
13. Walker-Samuel, S. et al. In vivo imaging of glucose uptake and metabolism in tumors. Nature medicine 19, 1067-1072, (2013).
14. Chan, K. W. et al. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magn Reson Med 68, 1764-1773, (2012).
15. Zaiss, M. et al. Inverse Z-spectrum analysis for spillover-, MT-, and T1 -corrected steady-state pulsed CEST-MRI--application to pH-weighted MRI of acute stroke. Nmr Biomed 27, 240-252, (2014).
16. Perevyazko, I. Y. et al. Polyelectrolyte complexes of DNA and linear PEI: formation, composition and properties. Langmuir 28, 16167-16176, (2012).
17. Koshkina, N. V., Agoulnik, I. Y., Melton, S. L., Densmore, C. L. & Knight, V. Biodistribution and pharmacokinetics of aerosol and intravenously administered DNA-polyethyleneimine complexes: Optimization of pulmonary delivery and retention. Mol Ther 8, 249-254, (2003).
18. Lin, E. H. et al. Polyethyleneimine and DNA nanoparticles-based gene therapy for acute lung injury. Nanomed-Nanotechnol 9, 1293-1303, (2013).
19. Ito, T., Yoshihara, C., Hamada, K. & Koyama, Y. DNA/polyethyleneimine/hyaluronic acid small complex particles and tumor suppression in mice. Biomaterials 31, 2912-2918, (2010).
Figures