0375
Inhomogeneous Magnetization Transfer (ihMT) imaging of the human brain at 7T1Aix Marseille Univ, CNRS, CRMBM, Marseille, France, 2APHM, Hôpital Universitaire Timone, CEMEREM, Marseille, France, 3Siemens Healthcare SAS, Saint-Denis, France, 4Aix-Marseille Univ, Université Gustave Eiffel, LBA, Marseille, France, 5iLab-Spine International Associated Laboratory, Marseille - Montreal, France, 6Division of MR Research, Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, United States
Synopsis
Inhomogeneous Magnetization Transfer (ihMT) MRI has raised interest for myelin imaging. Applications at ultra-high field are appealing for SNR considerations but are challenged by the reduction of available RF power due to regulatory SAR constraints and the need to account for inhomogeneities of the RF excitation field (B1+). In the current study we present experimental data acquired at 3T which validate two B1+-bias removal strategies. Then we further evaluate them at 7T and show preliminary results towards a robust ihMT protocol free from B1+ bias.
Introduction
Inhomogeneous Magnetization Transfer (ihMT) is a recent MRI modality1,2 that is sensitive and highly specific to myelination in central nervous system (CNS) tissues3 and has raised interest for clinical applications4–6. Following the initial developments of a clinical ready protocol for whole brain imaging at 1.5T7, further studies have enable human brain applications at 3T8–12. However, several challenges occur when moving towards higher field strengths, including reduction of available RF power due to regulatory SAR constraints and the inhomogeneity of the RF excitation field (B1+) when the size of the investigated volume of interest becomes comparable to the wavelength of the excitation field. While optimized RF saturation strategies7,13 allow reliable ihMT signal measurement in CNS tissues with RF powers available in vivo at 3T and 7T, mitigating the effects of RF non-uniformity is a key issue for ready-to-use ihMT protocols in a clinical setting.To date two approaches have been proposed to achieve this goal: i) a model free approach, based on the ihMT ratio (ihMTR) metric, exploiting the fact that the ihMTR signal reaches a plateau above a certain value of the power-averaged B1 (B1RMS), hence providing immunity to B1+ variations above a certain threshold12, and ii) a model-based approach relying on the ihMTsat metric11,14, adapted from the MTsat framework initially developed for conventional MT15. The ihMTsat metric includes a correction of T1 relaxation effects occurring within the various sequence delays. For given experimental conditions a specific B1+ dependence law may be assumed to further correct for RF non-uniformities. This approach then provides an ihMT metric less biased by T1 and B1+ effects. In the current study we present experimental data obtained at 3T to validate both approaches. The same strategies were evaluated at 7T and preliminary results lead the way towards an optimized protocol free from B1+ bias.
Methods
All experiments were performed on healthy volunteers under the guidelines of the institutional committee on clinical investigations. At 3T (MAGNETOM Vida, Siemens Heathcare), body coil transmission was combined with a 32-channel receive-only head coil. In contrast, at 7T (MAGNETOM Terra, Siemens Heathcare) a dedicated single-transmit and 32-channel receive head coil (Nova Medical) was used in combination with dielectric pads (7TSC, Multiwave Imaging). Both systems provide similar peak B1 amplitude (around 23 µT for a typical human brain load) but the B1RMS is more limited at 7T because of SAR regulatory constraints.The first approach based on the ihMTR metric was demonstrated with a prototype ihMT-GRE sequence, consisting of short trains of frequency alternated saturation pulses (for dual-frequency saturation) interleaved with multiple gradient echo readouts7. The sequence was run based on a protocol optimized for 3T which concentrates the MT pulse energy in time to reduce the sensitivity to B1+ variations and the ihMTR was computed as previously described12.
The ihMTsat approach was demonstrated on a prototype ihMT prepared RAGE sequence. In contrast with the previous implementation this ihMT sequence uses a different readout and a single cosine modulated pulse to achieve the dual-frequency saturation. A protocol previously optimized for 3T 8 was used, and for which a B1² dependency applies11, allowing correction of B1+ bias in ihMTsat. ihMTsat was calculated as in 11, using quantitative T1 and B1+ maps extracted from MP2RAGE16,17 and presaturated turboFLASH18 protocols, respectively (https://github.com/lsoustelle/ihmt_proc).
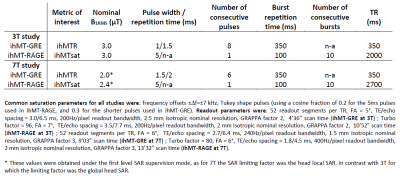
At 3T both sequences were run twice: once using nominal transmit voltage settings (Vref,100%), and then using transmit voltage deliberately attenuated by 20% (Vref,80%) to mimic B1+ variations over the whole brain12. Sequence parameters for all tested protocols at 3T and 7T are listed in Table 1.
Results
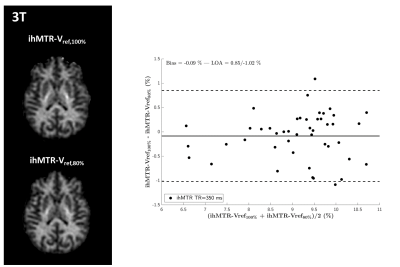
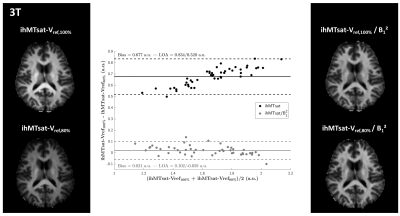
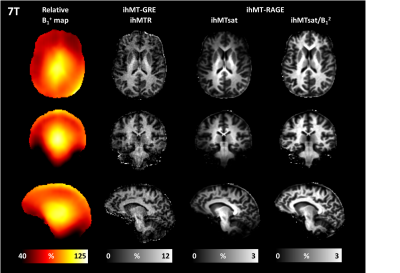
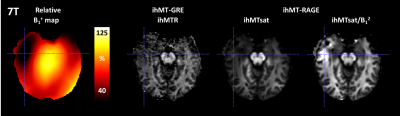
The 3T results are shown in Figures 1 and 2 and demonstrate good immunity to B1+ variations for both protocols, illustrated by relatively low biases and limits-of-agreement between the Vref,100% and the Vref,80% acquisitions in the Bland-Altman analyses performed on white matter regions-of-interest extracted from the JHU tract atlas19. Note that the ihMTR-GRE protocol provides a self-corrected ihMTR metric whereas the ihMTsat derived from the ihMT-RAGE protocol require a B1² correction to reach a good immunity.Results of the 7T acquisitions are displayed on Figure 3. The ihMTR metric displays a rather homogeneous contrast although signs of B1+ bias are perceived as a signal gradient from the center to the periphery of the brain. On the ihMTsat images the bias is more obvious and correlates well with the measured B1+ distribution. In contrast, once the B1² correction is applied the ihMTsat images recover a much more homogeneous contrast. Note however that both approaches fail to provide a robust signal quantification in brain areas of strong B1+ hypointensities (see Figure 4), suggesting that ihMTsat does not follow a quadratic B1 dependency in this regime.
Discussion and conclusion
To our knowledge these are the first in vivo ihMT images acquired at 7T on the human brain. Feasibility of ihMT at 7T was made possible by previous developments made at lower field strengths that address SAR constraints (e.g. low-duty cycle MT pulses and partial Fourier saturation7) and developing B1+ bias free ihMT acquisition strategies8,11,12. While these results are promising and emphasize the potential of moving toward ultra-high field, there is room for improvement to reach a more robust ihMT protocol at 7T.Acknowledgements
This work was supported by the SATT Sud-Est (France), the French Association pour la Recherche sur la Sclérose En Plaques (ARSEP 2020), the French National Research Agency, ANR [ANR-17-CE18-0030], and the Excellence Initiative of Aix-Marseille University - A*MIDEX [ANR-11-IDEX-0001-02].
The authors thank S. Confort-Gouny, V. Gimenez, P. Viout, L. Pini and C. Costes for technical support and management.
References
1. Varma G, Duhamel G, de Bazelaire C, Alsop DC. Magnetization Transfer from Inhomogeneously Broadened Lines: A Potential Marker for Myelin. Magn. Reson. Med. 2015;73:614–622 doi: 10.1002/mrm.25174 SMASH.
2. Girard OM, Prevost VH, Varma G, Cozzone PJ, Alsop DC, Duhamel G. Magnetization transfer from inhomogeneously broadened lines (ihMT): Experimental optimization of saturation parameters for human brain imaging at 1.5 Tesla. Magn. Reson. Med. 2015;73:2111–2121 doi: 10.1002/mrm.25330 SMASH.
3. Duhamel G, Prevost VH, Cayre M, et al. Validating the sensitivity of inhomogeneous magnetization transfer (ihMT) MRI to myelin with fluorescence microscopy. NeuroImage 2019 doi: 10.1016/j.neuroimage.2019.05.061 SMASH.
4. Van Obberghen E, Mchinda S, le Troter A, et al. Evaluation of the Sensitivity of Inhomogeneous Magnetization Transfer (ihMT) MRI for Multiple Sclerosis. AJNR Am. J. Neuroradiol. 2018 doi: 10.3174/ajnr.A5563 SMASH.
5. Zhang L, Wen B, Chen T, et al. A comparison study of inhomogeneous magnetization transfer (ihMT) and magnetization transfer (MT) in multiple sclerosis based on whole brain acquisition at 3.0 T. Magn. Reson. Imaging 2020;70:43–49 doi: 10.1016/j.mri.2020.03.010 SMASH.
6. Hou G, Lai W, Jiang W, et al. Myelin deficits in patients with recurrent major depressive disorder: An inhomogeneous magnetization transfer study. Neurosci. Lett. 2021;750:135768 doi: 10.1016/j.neulet.2021.135768 SMASH.
7. Mchinda S, Varma G, Prevost VH, et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: Sensitivity enhancement within a steady-state gradient echo sequence. Magn. Reson. Med. 2018;79:2607–2619 doi: 10.1002/mrm.26907 SMASH.
8. Varma G, Munsch F, Burns B, et al. Three-dimensional inhomogeneous magnetization transfer with rapid gradient-echo (3D ihMTRAGE) imaging. Magn. Reson. Med. 2020;84:2964–2980 doi: 10.1002/mrm.28324 SMASH.
9. Ercan E, Varma G, Mädler B, et al. Microstructural correlates of 3D steady-state inhomogeneous magnetization transfer (ihMT) in the human brain white matter assessed by myelin water imaging and diffusion tensor imaging. Magn. Reson. Med. 2018;80:2402–2414 doi: 10.1002/mrm.27211 SMASH.
10. Geeraert BL, Lebel RM, Mah AC, et al. A comparison of inhomogeneous magnetization transfer, myelin volume fraction, and diffusion tensor imaging measures in healthy children. NeuroImage 2018;182:343–350 doi: 10.1016/j.neuroimage.2017.09.019 SMASH.
11. Munsch F, Varma G, Taso M, et al. Characterization of the cortical myeloarchitecture with inhomogeneous magnetization transfer imaging (ihMT). NeuroImage 2021;225:117442 doi: 10.1016/j.neuroimage.2020.117442 SMASH.
12. Soustelle L, Troalen T, Hertanu A, et al. A strategy to reduce the sensitivity of inhomogeneous Magnetization Transfer (ihMT) imaging to radiofrequency transmit field variations at 3 T. Magn. Reson. Med. 2021 doi: 10.1002/mrm.29055 SMASH.
13. Varma G, Girard OM, Mchinda S, et al. Low duty-cycle pulsed irradiation reduces magnetization transfer and increases the inhomogeneous magnetization transfer effect. J. Magn. Reson. 2018;296:60–71 doi: 10.1016/j.jmr.2018.08.004 SMASH.
14. Rowley CD, Campbell JSW, Wu Z, et al. A model-based framework for correcting B 1 + inhomogeneity effects in magnetization transfer saturation and inhomogeneous magnetization transfer saturation maps. Magn. Reson. Med. 2021;86:2192–2207 doi: 10.1002/mrm.28831 SMASH.
15. Helms G, Dathe H, Kallenberg K, Dechent P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn. Reson. Med. 2008;60:1396–1407 doi: 10.1002/mrm.21732 SMASH.
16. Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele P-F, Gruetter R. MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. NeuroImage 2010;49:1271–1281 doi: 10.1016/j.neuroimage.2009.10.002 SMASH.
17. Van de Moortele P-F, Auerbach EJ, Olman C, Yacoub E, Uğurbil K, Moeller S. T1 weighted brain images at 7 Tesla unbiased for Proton Density, T2* contrast and RF coil receive B1 sensitivity with simultaneous vessel visualization. NeuroImage 2009;46:432–446 doi: 10.1016/j.neuroimage.2009.02.009 SMASH.
18. Chung S, Kim D, Breton E, Axel L. Rapid B1+ mapping using a preconditioning RF pulse with TurboFLASH readout. Magn. Reson. Med. 2010;64:439–446 doi: 10.1002/mrm.22423 SMASH.
19. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage 2008;40:570–582 doi: 10.1016/j.neuroimage.2007.12.035 SMASH.
Figures