0374
MR-Thermometry based targeting for histotripsy treatments in ex-vivo tissues1University of Michigan, Ann Arbor, MI, United States, 2Brigham Young University, Provo, UT, United States
Synopsis
The accuracy of using MR-Thermometry for pre-treatment targeting for histotripsy was evaluated on ex-vivo tissues. Eight brain samples were treated with an ultrasound array capable of performing both heating and histotripsy treatments. Heating was performed at array focus while MR-Thermometry images were acquired. Subsequently, histotripsy lesions were also made at the focus and post-treatment images were acquired. The location of MR-Thermometry estimated focal-zone was compared with the observed histotripsy lesion location to assess the accuracy of MR-Thermometry targeting. The mean error in estimated focus was about 1mm in all axes, suggesting MR-Thermometry would be suitable for pre-treatment targeting for histotripsy.
INTRODUCTION
Histotripsy is a focused ultrasound (FUS) therapy that uses cavitation to mechanically fractionate tissues into acellular debris1,2. Recent feasibility studies have demonstrated histotripsy to be a great candidate for non-invasive in-vivo brain treatment3,4,5. MR-guided histotripsy has been investigated for a wide range of brain applications. Accurate pre-treatment targeting is crucial to ensure that the brain treatment is carried out in the desired location, especially due to the presence of the skull in the beam path, which may cause the focal spot to shift from the geometric focus. In this study, we investigate using MR-Thermometry as a guide for histotripsy targeting. FUS is first used to generate low heating (<2C) at the ultrasound focus, which is visualized by MR-Thermometry and aligned with the histotripsy target. MR-Thermometry has been used to guide FUS thermal treatment in the brain6. Histotripsy uses very high focal pressure and is a non-linear, threshold phenomenon with negligible heating, while FUS-based low heating uses low pressure and is linear. Thus, the accuracy of using MR-Thermometry for targeting histotripsy treatment needs to be investigated.METHODS
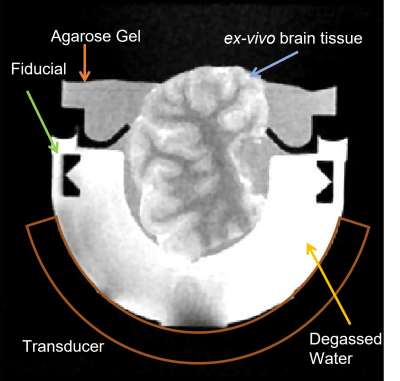
All experiments were performed in a 3T human scanner (GE MR750) with a 32-channel receive coil using a custom-built, 15-channel, 750kHz MRI-compatible ultrasound array (Fig. 1). A set of fiducial markers were placed on the array, such that the mid-point estimated from the two fiducials approximates the geometric focus. Since the drivers were capable of transmitting short pulses (~6μs) only, we used a high duty cycle and low-pressure pulses for heating and a low duty cycle and high negative pressure pulses for histotripsy (Fig. 2).Heating was performed by using a 50kHz pulse repetition frequency (PRF) (30% duty cycle) at an estimated 8.1MPa peak-peak pressure in free-field to heat the tissue to a few degrees above room temperature. The low peak-peak pressure ensured that the probability of cavitation around the treatment regions was low. Since the focal distance was 5cm and the peak pressure was low, non-linear propagation effects were also minimized. The tissues were heated for 15seconds while simultaneous MR-Thermometry images were acquired using an RF spoiled GRE with a spiral acquisition. GRE scan treatment parameters were: TE/TR:30ms/1000ms, 13cm FOV, 2mm slice, 128x128 matrix, and 4 shots. Image reconstruction was done using conjugate phase reconstruction and Fourier interpolation was done to increase the matrix size to 256x256. Drift correction was applied on phase difference images by subtracting the mean phase of a non-heated region from the whole image.
Histotripsy lesions of 2.5mm by 3mm in transverse and longitudinal planes, respectively, were created centered at the geometric focus of the array. 100 histotripsy pulses were delivered to each treatment location within the treatment volume using a PRF of 50Hz (0.03% duty cycle) and a peak negative pressure of about -54MPa. Post-treatment T2, T2*, and DWI (b=1000s/mm2) scans were used to estimate the location of the focus. All scans were acquired with the same scan prescription. DWI and GRE were acquired with the same spiral acquisition and reconstruction to ensure accurate alignment between the two.
RESULTS
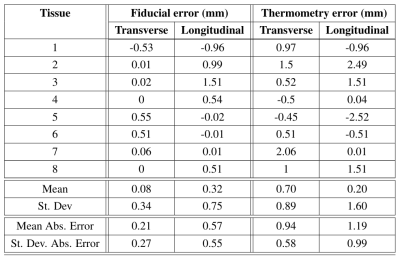
To estimate the focus from heating images, an ROI that contained the heated region was selected and a 2D Gaussian was fitted to the temperature values. The peak of the Gaussian was estimated as the focus (Fig. 3). To avoid thermal diffusion affecting the location of the estimated focus, the fit was calculated from the earliest image where temperature change was easily observable. In all the tissues, no contrast was seen in T2* images, while for some tissues, T2 images showed the lesion. DWI images were able to visualize the lesion for all tissues (Fig. 4). Due to partial-volume effects, it was expected to see the lesion visualized in different numbers of voxels for each sample. To estimate the histotripsy focus from these images, the voxel with the most hypo-intense spot in DWI was designated as the focus. Table 1 shows the distance between the histotripsy lesion and the focus estimated from fiducials and MR-Thermometry. For thermometry-based targeting, the mean absolute error in the estimated focus is 0.92mm and 1.19mm in transverse and longitudinal directions, respectively. No bias in the thermometry estimated focus is seen. However, histotripsy focus estimation using fiducial is better than thermometry for all the tissues.CONCLUSION AND DISCUSSION
This study demonstrates the feasibility of using MR-Thermometry for pre-treatment targeting in MR-guided histotripsy experiments. In the ideal case tested with short distance and no severe aberration, MR-Thermometry can estimate histotripsy focus with about 1mm accuracy in both axes. With an image resolution of 0.5mm, this result is at the limits of the resolving power provided by MRI. Since histotripsy lesions are elongated along the direction of beam propagation, some inaccuracy in estimation in that axis may be expected. Also, partial volume effects in the DWI images make the focus estimation more difficult since more than 1 voxel can be designated as focus. Although fiducials perform better in these experiments, they will be less useful when the lesions are created with focal steering, or when facing severe aberration. Future work will look at the effect of steering, aberration, and also non-linear wave propagation on accuracy of the thermometry estimated focus.Acknowledgements
This work is supported by NIH Grant R01EB028309.References
[1] Xu, Z., Ludomirsky, A., Eun, L. Y., Hall, T. L., Tran, B. C., Fowlkes, J. B., & Cain, C. A. (2004). Controlled ultrasound tissue erosion. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control, 51(6), 726–736.
[2] Parsons, J. E., Cain, C. A., Abrams, G. D., & Fowlkes, J. B. (2006). Pulsed cavitational ultrasound therapy for controlled tissue homogenization. Ultrasound in Medicine and Biology, 32(1), 115–129.
[3] Sukovich, J. R., Cain, C. A., Pandey, A. S., Chaudhary, N., Camelo-Piragua, S., Allen, S. P., Hall, T. L., Snell, J., Xu, Z., Cannata, J. M., Teofilovic, D., Bertolina, J. A., Kassell, N., & Xu, Z. (2019). In vivo histotripsy brain treatment. Journal of Neurosurgery, 131(4), 1331–1338.
[4] Lu, N., Gupta, D., Daou, B. J., Fox, A., Choi, D., Sukovich, J. R., Hall, T. L., Camelo-Piragua, S., Chaudhary, N., Snell, J., Pandey, A. S., Noll, D. C., & Xu, Z. (2021). Transcranial Magnetic Resonance-Guided Histotripsy for Brain Surgery: Pre-clinical Investigation. Ultrasound in Medicine & Biology, 00(00), 1–13.
[5] Allen, S. P., Vlaisavljevich, E., Shi, J., Hernandez-Garcia, L., Cain, C. A., Xu, Z., & Hall, T. L. (2017). The response of MRI contrast parameters in in vitro tissues and tissue mimicking phantoms to fractionation by histotripsy. Physics in Medicine and Biology, 62(17), 7167–7180.
[6] Rieke, V., & Pauly, K. B. (2008). MR thermometry. Journal of Magnetic Resonance Imaging 27(2), 376–390
Figures