0362
Shuttered Echo Planar fMRI with Dynamic Motion and Phase Correction1Vanderbilt University Institute of Imaging Science, Nashville, TN, United States, 2Department of Radiology, Vanderbilt University Medical Center, Nashville, TN, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 4Department of Radiology, Harvard Medical School, Boston, MA, United States, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States, 6Radiological Sciences Laboratory, Stanford University, Standford, CA, United States, 7Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States
Synopsis
Achieving submillimeter-resolution BOLD fMRI is a challenge since single-shot EPI suffers from long echo times, blurring, and image distortions, while multishot EPI suffers from motion- and respiration-induced shot-to-shot phase errors. In shuttered multishot EPI, data are acquired in each shot after exciting a set of interleaved “shutters” across the imaged slice. This enables high quality reconstructions from each shot, which in turn enables navigator-free shot-wise phase and motion corrections prior to reconstructing a full-FOV image. Herein we describe a full motion- and phase-correcting image reconstruction for dynamic shuttered EPI and validate it in head motion and BOLD fMRI experiments.
Introduction
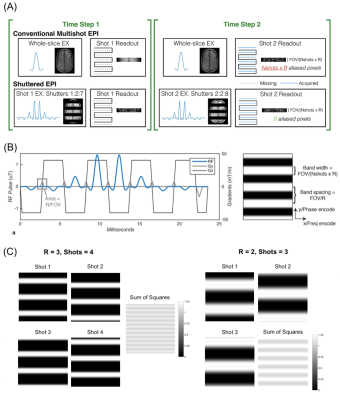
In recent years, considerable effort has been made to achieve sub-millimeter resolution echo planar imaging (EPI) of the brain1-3. However, single-shot EPI under current hardware limitations suffers from long echo times and blurring at sub-millimeter resolutions. Multishot EPI4 enables high-resolution imaging with low blurring but is vulnerable to motion and respiration-induced shot-to-shot phase variations5,6. We previously described a Shuttered multishot EPI pulse sequence at 7 Tesla (Figure 1A) for self-navigated phase- and motion-robust imaging at high resolution7. The technique has also been applied to diffusion MRI of the breast and the brain8,9. Here we describe a GRAPPA-based dynamic motion- and phase-correcting Shuttered EPI image reconstruction and evaluate it in head motion and sub-millimeter in-plane BOLD fMRI experiments at 7 Tesla.Methods
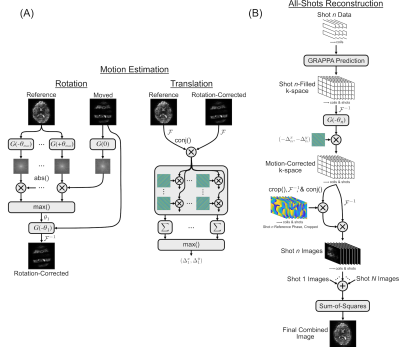
Shuttered EPI sequence: In Shuttered EPI, a 2D RF pulse (Figure 1B) excites a set of shutters of width FOV/(R x Nshots) across the image plane, instead of the entire FOV. Shot-wise phase increments in the RF pulse are used to shift the shutters and cover the full FOV. This results in an effective reduction factor of R for each shot, facilitating high-SNR reconstructions that can be used to determine shot-wise motion and phase corrections.Motion- and Phase-Corrected Image Reconstruction: Figure 2 shows the pipelines for dynamic Shuttered EPI motion estimation and reconstruction10. The individual-shot and all-shot reconstructions used GRAPPA kernels trained from ACS data obtained in Nshots separate unaccelerated scans with each shot’s corresponding 2D RF pulse, with echo train lengths and ordering matched to the accelerated dynamic scan.
Shot-Wise Motion Estimation (Figure 2A)11: To estimate in-plane rigid body motion parameters, individual shot images were reconstructed using their own GRAPPA kernels, and Fourier-transformed and correlated with a library of rotated reference image k-space amplitudes to extract shot-wise in-plane rotations11. The rotations were corrected, and translations are estimated by complex correlation with a library of translated reference image k-spaces.
All-Data Phase-Corrected Reconstruction (Figure 2B): The all-shots GRAPPA kernel was used to calculate each shot’s contributions to its and the other shots’ k-spaces. These k-spaces were motion-corrected using the estimated parameters for each shot, and inverse Fourier-transformed. Low-resolution phase difference maps were calculated between the resulting images and reference images and subtracted. After phase correction the complex images were summed across shots, and the final image was obtained by root-sum-of-squares combination of images across coils and shots.
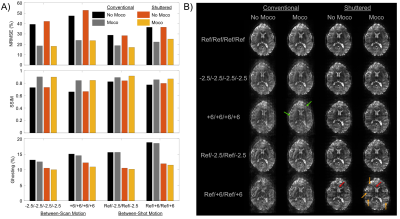
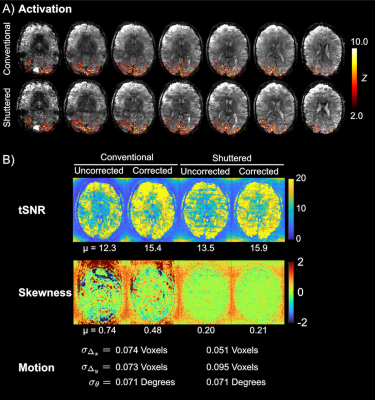
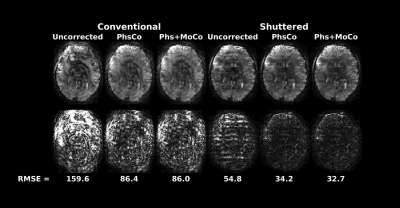
Experiments: Two adult human experiments were performed with IRB approval in a 7 Tesla scanner (Philips Healthcare, The Netherlands) with a dual-transmit, 32-channel receive coil setup. The first experiment evaluated motion correction with parameters: 228 x 228 mm2 FOV, 1 x 1 x 4 mm3, 15 slices, R = 3, 4 shots, and TR/TE 3000/27 ms. Motion between shots was simulated by acquiring scans in three different head positions and retrospectively mixing shot data. Reconstructed image volumes were evaluated for image quality in terms of NRMSE and SSIM compared to motion-free reconstruction, and ghosting computed as the ratio of the RMS signal outside the brain versus the signal inside. The second experiment was a BOLD fMRI scan with parameters: 220 x 220 mm2 FOV, 0.8 x 0.8 x 4 mm3, 7 slices, R = 4, 4 shots, volume TR/TE of 2000/28 ms and 150 dynamics. All scans used a flip angle of 78 degrees. The block design paradigm consisted of a 16 s on/36 s off, 8 Hz flashing checkerboard stimulus presented for 312 s. Matched conventional multishot scans were also collected. All reconstructions (Shuttered and Conventional) used the method described above with the same settings. Activation maps were derived using standard GLM analysis in FSL FEAT12. Image quality of timeseries data was evaluated with temporal SNR (tSNR) and temporal skewness13, which is sensitive to the degree of intermittent ghosting in time series data.
Results
Figure 3 shows that motion correction improved image quality both for Conventional and Shuttered EPI. However, the Shuttered images showed significantly lower ghosting than Conventional images, both for intra- and inter-shot motion. Figure 4A shows BOLD fMRI activation maps, which were consistent with expected responses to visual stimuli for both scans, though there were fewer spurious activations in the front of the brain in Shuttered EPI. The scans had comparable tSNR over the 7-slice volume (15.4 for Conventional and 15.9 for Shuttered). However, temporal skewness was significantly higher for Conventional multishot (0.48 compared to 0.21), reflecting much higher intermittent ghosting in that scan, most likely arising from respiration. Figure 5 shows a video of the 10 time points with the highest RMSEs referenced to the mean image for each technique. Conventional EPI images contain large ghosts related to respiration, which are significantly reduced in Shuttered EPI.Discussion
A phase- and motion-corrected image reconstruction for Shuttered EPI was described and validated in head motion and fMRI experiments, which enabled phase- and motion-robust submillimeter-resolution dynamic EPI with low ghosting. The same reconstruction was also applied to Conventional multishot EPI, where it yielded similar improvements and could thus be used for self-navigated Conventional multishot reconstruction, though higher ghosting remained compared to Shuttered EPI. Future work will combine Shuttered EPI with SMS for further scan acceleration, and pTx to reduce pulse durations.14,15Acknowledgements
This work was supported by NIH R01 EB 016695 and R01 EB 019437.References
1. De Martino F, Yacoub E, Kemper V, et al. The impact of ultra-high field MRI on cognitive and computational neuroimaging. Neuroimage. 2018;168:366-382.
2. Dumoulin SO, Fracasso A, van der Zwaag W, et al. Ultra-high field MRI: Advancing systems neuroscience towards mesoscopic human brain function. Neuroimage. 2018;168:345-357. PMID: 28093360.
3. Setsompop K, Feinberg DA, Polimeni JR. Rapid brain MRI acquisition techniques at ultra-high fields. NMR Biomed. 2016;29(9):1198-1221. PMID: 26835884.
4. McKinnon GC Magn Reson Med. 1993; 30, 609-616.
5. Polimeni JR et al, Magn Reson Med. 2016; 75(2), 665-679.
6. Zur Y. Magn Reson Med, 66(6). 2011; 1616-1626
7. Sengupta S, Setsompop K, Grissom WA. Shuttered EPI Brain Imaging at 7 Tesla. In: Proceedings of the 26th Annual Meeting of ISMRM. Paris, France; 2018. p. 0216.
8. Taviani V, Alley MT, Banerjee S, et al . High-resolution diffusion-weighted imaging of the breast with multiband 2D radiofrequency pulses and a generalized parallel imaging reconstruction. Magn Reson Med. 2017 Jan;77(1):209-220. doi: 10.1002/mrm.26110. PMCID: PMC8158261.
9. Sun K, Zhong Z, Xu Z, et al. In-plane simultaneous multisegment imaging using a 2D RF pulse. Magn Reson Med. 2021; Aug 4. doi: 10.1002/mrm.28956.. PMID: 34350601.
10. Griswold MA, Jakob PM, Heidemann RM, et al Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med. 2002 Jun;47(6):1202-10. doi: 10.1002/mrm.10171. PMID: 12111967.
11. Lin W, Huang F, Börnert P, et al . Motion correction using an enhanced floating navigator and GRAPPA operations. Magn Reson Med. 2010 Feb;63(2):339-48. doi: 10.1002/mrm.22200. PMID: 19918907.
12. Woolrich MW, Ripley BD, Brady M, et al . Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370-1386.
13. Berman AJL, Grissom WA, Witzel T, et al . Ultra-high spatial resolution BOLD fMRI in humans using combined segmented-accelerated VFA-FLEET with a recursive RF pulse design. Magn Reson Med. 2021 Jan;85(1):120-139. doi: 10.1002/mrm.28415. PMCID: PMC7722122.
14. Feinberg DA, Setsompop K. Ultra-fast MRI of the human brain with simultaneous multi-slice imaging. J Magn Reson. 2013 Apr; 229:90-100. doi: 10.1016/j.jmr.2013.02.002. PMCID: PMC3793016.
15. Cao Z, Yan X, Grissom WA, Parallel Transmit Excitation Pulses for Shuttered Echo Planar Imaging. In: Proceedings of the 26th Annual Meeting of ISMRM. Paris, France; 2018. p. 0386
Figures