0360
NeuroMix - A motion robust single scan brain exam1GE Healthcare, Stockholm, Sweden, 2Karolinska Institutet, Stockholm, Sweden, 3Uppsala University Hospital, Uppsala, Sweden, 4Karolinska University Hospital, Stockholm, Sweden
Synopsis
We have presented a new multi-contrast sequence, NeuroMix, which acquires T1w, T2w, T2-FLAIR, T2*w, and DWI contrasts in a single scan, requiring only one prescription and one prescan. NeuroMix includes single-shot EPI and FSE readouts as well as optional multi-shot FSE and 3DEPI acquisitions. The motion robustness of NeuroMix’s single-shot contrasts was generally good even when NeuroMix’s multi-shot sequences were unusable due to motion corruption resulting in a high probability to obtain diagnostic images. In conclusion, NeuroMix enables a fast and motion robust single-scan brain exam allowing higher patient throughput with less discomfort and possibly less need for sedation.
Introduction
We implemented a fast, motion robust pulse sequence called NeuroMix that acquires the most important brain imaging contrasts T1w, T2w, T2*w, T2-FLAIR, and DWI(1) in one run with only one prescription and one prescan. The order of the contrasts is carefully optimized and some contrasts are acquired in the transient state (longitudinal magnetization) to save additional scan time. Motion robust single-shot readouts of all five contrasts are combined with optional, higher resolution but less motion robust multi-shot readouts. Compared to its predecessor EPIMix (2), NeuroMix includes not only EPI but also distortion-free FSE readouts. We envision NeuroMix to be used as a standalone single-scan brain screening MRI exam.Methods
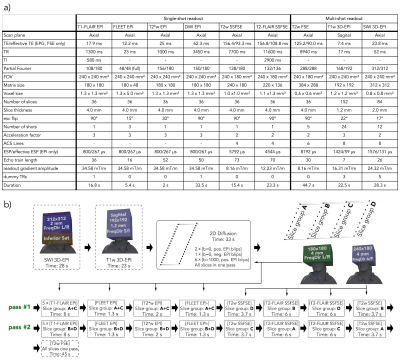
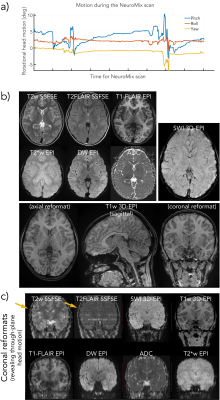
A software framework based on KSFoundation (3) was developed that configures and runs several sequences in one main sequence. The sequence parameters of all subsequences are overviewed in Figure 1a. NeuroMix includes five single-shot contrasts based on 2DEPI and SSFSE and three optional multi-shot contrasts that include a high-resolution T2w FSE, a high-resolution 3D-EPI SWI as well as a 3D-EPI T1w acquisition with isotropic resolution. NeuroMix is customizable and takes between 1:20 min and 4 min for a full brain exam. NeuroMix for this work included T1FLAIR EPI with 5 averages, DWI with six directions, a reverse polarity b=0 volume, FLEET calibration for both EPI polarities, and all optional contrasts resulting in a scan time of 3:32 min. Figure 2b illustrates the FOV and the acquisition order of all contrasts. All single-shot contrasts except the DWI EPI are acquired in two passes to allow wider inversion slices for T1FLAIR and T2FLAIR. The slices of T2w SSFSE and T2FLAIR SSFSE are grouped into four stacks to enable a transient state acquisition without dummy cycles.In total, four scans were acquired where a healthy volunteer was first instructed to hold still and then to carry out three different continuous motion patterns, small left-right rotation and large left-right rotation (both in-plane) as well as nodding motion (through plane). The motion was recorded using a markerless optical tracking system (Tracoline TCL3.1m, research version provided by TracInnovations, Ballerup, Denmark) (4). Additionally, a pediatric patient was scanned using NeuroMix without the multi-shot T2w FSE.Results
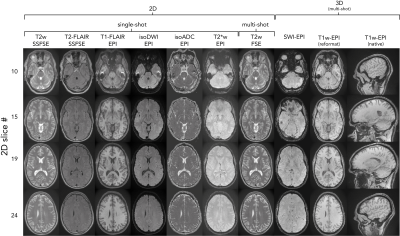
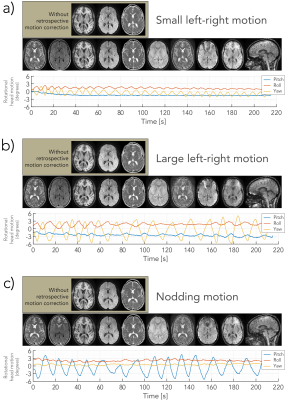
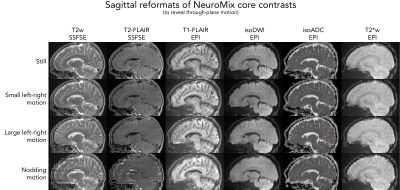
Fig. 2 depicts all contrasts of NeuroMix for the motion-free experiment. When compared to the 2D EPI readouts, the FSE sequences have reduced geometrical distortions particularly at the skull base (slice 10). Also, the 3DEPI sequences exhibit significantly reduced distortions owing to the smaller effective echo spacing (Table 1). The image quality of the multi-shot sequences dropped dramatically for small left-right motion and is unusable for large left-right and nodding motion (Fig. 3). On the contrary, the single-shot sequences achieved sharp images even under severe motion. However, nodding motion corrupted some image contrasts due to magnetization history effects. The CSF suppression in T2FLAIR SSFSE partially fails due to misalignment of the inversion and the imaging slice. This impression is confirmed by the sagittal reformats in Fig. 4. Small left-right motion resulted in similar image quality compared to the still acquisition. Under large left-right motion, slice-to-slice inconsistencies become apparent, especially in the region of the forehead. Finally, under nodding motion, significant image corruption can be seen in the sagittal view. T2w SSFSE shows signal drops in some slices due to magnetization history effects (saturation from previous slices). T2FLAIR exhibits false hyperintensities due to failed CSF suppression across the slice stack. Also, the sagittal image sharpness drops significantly while it is largely unaffected in axial. The pediatric patient scan provides a more realistic motion pattern (Fig. 5). Again, the single-shot contrasts seem to be of a high enough quality for diagnostic use with the only limitation being failed CSF suppression in some slices due to through-plane motion.Discussion
The distortion-free single-shot FSE readouts for T2w and T2-FLAIR and the optional multi-shot FSE and 3D-EPI contrasts significantly increased NeuroMix’s diagnostic value over its predecessor EPIMix which was based only on single-shot EPI. The scan-time of NeuroMix for full brain coverage ranges from 1:20min with single-shot only up to 4 min with all optional contrasts plus a higher number of (single-shot) averages. The motion robustness of NeuroMix’s single-shot contrasts was generally good and extended far beyond NeuroMix’s multi-shot sequences. This supports the idea of backing up high-quality multi-shot sequences with motion robust single-shot sequences. Throughplane motion remains a problem as it can corrupt the image contrast, particularly for T2FLAIR. However, even in these cases, NeuroMix provided sharp images in the native acquisition plane. Prospective motion correction seems the best way to further improve motion robustness. In conclusion, NeuroMix enables a fast and motion robust single-scan brain exam allowing higher patient throughput with less discomfort and possibly less need for sedation.Acknowledgements
No acknowledgement found.References
1. Mehan WA Jr, González RG, Buchbinder BR, Chen JW, Copen WA, Gupta R, et al. Optimal brain MRI protocol for new neurological complaint. PLoS One. 2014 Oct 24;9(10):e110803.
2. Skare S, Sprenger T, Norbeck O, Rydén H, Blomberg L, Avventi E, et al. A 1-minute full brain MR exam using a multicontrast EPI sequence. Magn Reson Med. 2018 Jun;79(6):3045–54.
3. Skare S, Avventi E, Norbeck O, Ryden H. An abstraction layer for simpler EPIC pulse programming on GE MR systems in a clinical environment. In: Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, Hawaii. 2017. p. 3813.
4. Frost R, Wighton P, Karahanoğlu FI, Robertson RL, Grant PE, Fischl B, et al. Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn Reson Med. 2019 Jul;82(1):126–44.
Figures