0357
Denoising task-correlated head motion in motor-task fMRI data using multi-echo ICA
Neha A. Reddy1,2, Rachael C. Stickland1, Kimberly J. Hemmerling1,2, Kristina M. Zvolanek1,2, César Caballero-Gaudes3, and Molly G. Bright1,2
1Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, United States, 2Biomedical Engineering, Northwestern University, Evanston, IL, United States, 3Basque Center on Cognition, Brain and Language, Donostia, Spain
1Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, United States, 2Biomedical Engineering, Northwestern University, Evanston, IL, United States, 3Basque Center on Cognition, Brain and Language, Donostia, Spain
Synopsis
Multi-echo independent component analysis (ME-ICA) has been shown to differentiate the effects of head motion from desired BOLD signal in fMRI data, but this method has not been tested in motor-task studies with high amounts of task-correlated head motion. We investigated four denoising models on multi-echo motor-task data with limited and amplified task-correlated motion: Aggressive, Moderate, and Conservative ME-ICA nuisance regression models and a conventional optimally combined (OC) model. ME-ICA models were found to better dissociate head motion and BOLD signal variance than the OC model. Among them, the Aggressive model had the most consistent activation results with amplified motion.
Introduction
Motor-task fMRI studies are often complicated by task-correlated motion that can confound imaging results. One method that has been shown to differentiate the effects of head motion from the desired BOLD signal is multi-echo independent component analysis (ME-ICA)1,2. However, this analysis technique has not been tested in motor-task studies with high amounts of task-correlated head motion, which is especially prevalent in motor-impaired populations3. In this study, we evaluate the use of ME-ICA denoising models in the analysis of two motor tasks: (1) with limited head motion and (2) with amplified task-correlated head motion to simulate a motor-impaired population.Methods
Data Collection: Two fMRI scans were collected from five healthy participants (2M,25±2y). Data were acquired on a Siemens 3T Prisma MRI system with a 64-channel head coil, using a multi-band gradient-echo planar imaging sequence: TR=2s, TEs=10.8/28.03/45.26/62.49/79.72ms, FA=70°, MB factor=4, voxel size=2.5x2.5x2mm3. Tape placed across the participants’ forehead provided tactile feedback of head motion4. During each scan, participants performed an isometric unimanual grasping task at 40% maximum grip strength: 10-s “squeeze” and 15-s “relax”, 8 repetitions/hand. Participants viewed instructions and real-time force feedback on a screen. During the first scan (MOTOR), participants were instructed to keep their head as still as possible. During the second scan (MOTOR+motion), participants were instructed to nod their head slightly during each grasp to add task-correlated motion.Data Analysis: FSL5 and AFNI6 tools were used for analysis. The first 10 volumes of each functional scan were removed to allow for steady-state magnetization. Head-motion realignment parameters were computed for the first echo with reference to the Single Band reference image taken at the start of the scan, then applied to all echoes. An optimally combined image was calculated, ME-ICA was performed (tedana7), and components were manually classified (accepted or rejected as noise8) aided by Rica9. Each dataset was processed with four models, similar to those used in Moia et al.2:
- An optimally combined (OC) model in which the design matrix included motion parameters from volume realignment, Legendre polynomials up to third order, and left- and right-hand grip tasks convolved with a hemodynamic response function,
- An Aggressive ME-ICA model that also included rejected ICA components orthogonalized with respect to motion parameters and Legendre polynomials,
- A Moderate ME-ICA model, in which rejected components from the Aggressive model were additionally orthogonalized with respect to left- and right-hand grip regressors, and
- A Conservative ME-ICA model, in which rejected components from the Moderate model were additionally orthogonalized with respect to ME-ICA accepted (non-noise) components.
For each model, the denoised signal was calculated by subtracting the product of the nuisance regressor beta-coefficient maps and their time series from the OC data. Framewise displacement was calculated from volume realignment parameters, and DVARS from each denoised 4D dataset10. Areas of significant activation for the right-hand grip task were defined as voxels with z-score>1.96 (p<0.05, False-Discovery-Rate-corrected). A left primary motor cortex (M1) mask11 was considered to examine activation in motor regions. We analyzed each model’s performance in areas of expected activation (M1) and noise (full brain) to investigate how the models deal with false positives and negatives caused by head motion.
Results
All participants performed the MOTOR+motion task with greater overall and task-correlated motion compared to the MOTOR task (Fig1, Tab1).During both tasks, DVARS was less related to FD in all ME-ICA models compared to OC. The ME-ICA models were not significantly different from each other in the MOTOR condition; however, in the MOTOR+motion condition, the Moderate and Aggressive models were significantly better than the Conservative model (Fig2).
On qualitative examination, all four models detected activation in M1 during both tasks. The Conservative and Moderate ME-ICA models resulted in greater activation (likely noise) outside this region when motion was added (Fig3). Only the Aggressive model was able to compensate for the typical stripe artifacts resulting from head displacements in multi-band acquisitions12,13. The Aggressive model led to the fewest positive activated voxels in M1 and negative activated voxels in the full brain; these voxel numbers were most consistent between task types. These results suggest the Aggressive model was least influenced by false positive and negative activation. The Moderate and Conservative models increased the number of activated voxels when task-correlated motion was present, and the OC model performed in-between the Aggressive and Moderate/Conservative models (Fig4).
Discussion and Conclusion
We were successful in simulating amplified task-correlated head motion during a motor task, with all participants exhibiting greater FD and task-correlated motion during the MOTOR+motion condition. Our analysis of the relationship between DVARS and FD demonstrates that ME-ICA models are an effective tool for dissociating head motion from desired BOLD effects2. The Aggressive and Moderate ME-ICA models, in particular, may have added utility in the setting of high task-correlated motion.Use of the Aggressive denoising model produced activation maps that contained minimal multi-band-related artifacts and were consistent across scans with varying degrees of task-correlated head motion. Combined with the DVARS results, this suggests that the Aggressive model is an adequate denoising model for use in motor-task studies with higher levels of task-correlated motion. However, this model should be used with caution since it also slightly reduces the activation in M1 compared to the conventional model.
Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness (Ramon y Cajal Fellowship, RYC-2017- 21845), the Basque Government (BERC 2018-2021 and PIBA_2019_104), and the Spanish Ministry of Science, Innovation and Universities (MICINN; PID2019-105520GB-100).References
- Kundu P, Inati SJ, Evans JW, Luh WM, Bandettini PA. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage 2012;60:1759–70. https://doi.org/10.1016/j.neuroimage.2011.12.028.
- Moia S, Termenon M, Uruñuela E, Chen G, Stickland RC, Bright MG, et al. ICA-based denoising strategies in breath-hold induced cerebrovascular reactivity mapping with multi echo BOLD fMRI. Neuroimage 2021;233:1–18. https://doi.org/10.1016/j.neuroimage.2021.117914.
- Seto E, Sela G, McIlroy WE, Black SE, Staines WR, Bronskill MJ, et al. Quantifying head motion associated with motor tasks used in fMRI. Neuroimage 2001;14:284–97. https://doi.org/10.1006/nimg.2001.0829.
- Krause F, Benjamins C, Eck J, Lührs M, van Hoof R, Goebel R. Active head motion reduction in magnetic resonance imaging using tactile feedback. Hum Brain Mapp 2019;40:4026–37. https://doi.org/10.1002/hbm.24683.
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. FSL. Neuroimage 2012;62:782–90. https://doi.org/10.1016/j.neuroimage.2011.09.015.
- Cox J.S. RW. H. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162–73.
- Dupre E, Salo T, Ahmed Z, Bandettini PA, Bottenhorn KL, Caballero-Gaudes C, et al. TE-dependent analysis of multi-echo fMRI with tedana. J Open Source Softw 2021;6:1–5. https://doi.org/10.21105/joss.03103.
- Griffanti L, Douaud G, Bijsterbosch J, Evangelisti S, Alfaro-Almagro F, Glasser MF, et al. Hand classification of fMRI ICA noise components. Neuroimage 2017;154:188–205. https://doi.org/10.1016/j.neuroimage.2016.12.036.
- Uruñuela E. Rica. GitHub Repository. 2021.
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012;59:2142–54. https://doi.org/10.1016/j.neuroimage.2011.10.018.
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. Neuroimage 2006;31:1453–74. https://doi.org/10.1016/j.neuroimage.2006.02.004.
- Olafsson V, Kundu P, Wong EC, Bandettini PA, Liu TT. Enhanced identification of BOLD-like components with multi-echo simultaneous multi-slice (MESMS) fMRI and multi-echo ICA. Neuroimage 2015;112:43–51. https://doi.org/10.1016/j.neuroimage.2015.02.052.
- Todd N, Moeller S, Auerbach EJ, Yacoub E, Flandin G, Weiskopf N. Evaluation of 2D multiband EPI imaging for high-resolution, whole-brain, task-based fMRI studies at 3T: Sensitivity and slice leakage artifacts. Neuroimage2016;124:32–42. https://doi.org/10.1016/j.neuroimage.2015.08.056.
Figures
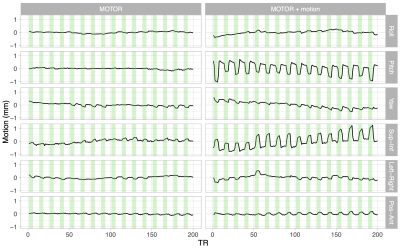
Figure 1. Demeaned head motion traces for Subject 1 during the MOTOR and MOTOR+motion tasks. Rotations in degrees were converted to millimeters using a spherical head model10. Green shaded areas represent periods when the subject was performing the grasping task. Mean and task-correlated motion were greater during the MOTOR+motion task, particularly in the pitch rotation and superior-inferior translation directions, showing the subject’s compliance with the task.
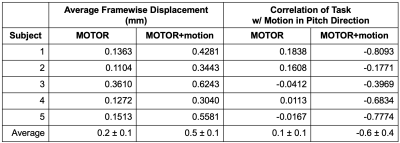
Table 1. Average Framewise Displacement (FD) and point-biserial correlation of the task with motion in pitch direction (head nod) for each functional scan. FD and task-correlated motion increased in the MOTOR+motion condition for every subject. Correlations were converted to Fisher’s z to calculate averages and are reported as Pearson’s r.
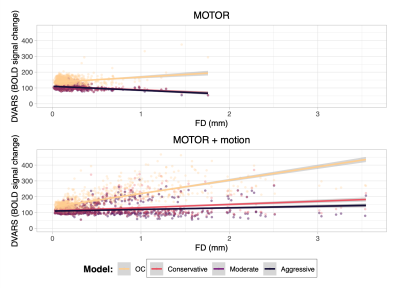
Figure 2. DVARS vs Framewise Displacement (FD) considering all subjects. For each task, data were modeled as DVARS ~ FD + Model + (1|Subject). For the MOTOR task, the three ME-ICA conditions were not significantly different from each other (overlapping fit line on graph). For the MOTOR+motion task, the Moderate and Aggressive ME-ICA models were not significantly different from each other (overlapping fit line on graph).
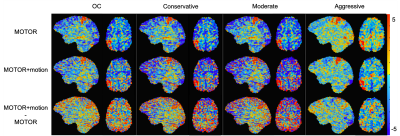
Figure 3. T-statistic maps of right-hand grip activation for Subject 1 during MOTOR and MOTOR+motion tasks. A difference map is shown to represent the change in t-statistic when task-correlated motion is added. All slices show the same sagittal and axial brain regions. Negative t-statistic stripe artifacts are noted in the OC, Conservative, and Moderate models in the MOTOR+motion condition.
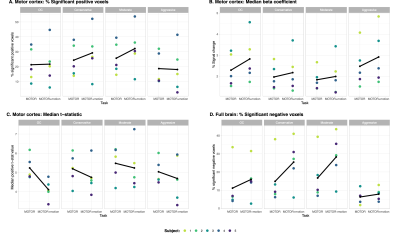
Figure 4. Percentage of significant positive voxels (A), median beta-coefficient (B), and median t-statistic (C) in M1 during right-hand grip (z-score>1.96, p-value<0.05, FDR-corrected). Percentage of significant negative voxels in the full brain during right-hand grip (D). Black lines represent the subject mean. Greater number of significant voxels in M1 during the MOTOR+motion task may be influenced by activation in the head/neck motor area due to the added voluntary head movement. Multi-band acquisition may also cause potential false positive activation13.
DOI: https://doi.org/10.58530/2022/0357