0333
Free-running contrast-enhanced ultra-short TE (UTE) for cardiac and respiratory motion-resolved flow artifact-free 5D whole-heart MRI1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2Advanced Clinical Imaging Technology (ACIT), Siemens Healthcare AG, Lausanne, Switzerland, 3CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 4Division of Cardiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 5Director CMR-Center, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 6Faculty of Biology and Medicine, University of Lausanne (UNIL), Lausanne, Switzerland, 7Division of Pediatric Cardiology, Woman-Mother-Child Department, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 8Service of Cardiology, Heart and Vessel Department, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland
Synopsis
Free-running whole-heart MRI can suffer from flow artifacts. Despite the efficiency of 3D radial UTE in minimizing the latter, its integration as a free-running sequence has so far been challenging due to poor-quality self-gating, which has necessitated an inefficient dual-echo approach. In this work we show that self-gating signals from a single-echo ferumoxytol-enhanced free-running 3D radial UTE sequence are comparable to the dual-echo approach, allowing to significantly improve scanning efficiency and produce dynamic images that are free from flow artifacts.
Introduction
In the free-running framework for whole-heart MRI, data are continuously acquired independently from the underlying physiological motion, and retrospectively reconstructed as cardiac and respiratory motion-resolved 5D images using self-gating (SG) signals derived from the data themselves1. In addition to providing high-quality dynamic visualizations of cardiac anatomy, this approach meets the need for a simplified and time-efficient workflow for cardiac MR exams. In this context, ferumoxytol-enhanced 5D imaging with a 3D radial gradient echo (GRE) sequence has been shown to provide excellent delineation of cardiac anatomy by enhancing the blood-pool signal2,3. Nonetheless, dephasing effects due to blood flow in concert with shortened T2* can produce artifactual signal voids adversely affecting the image quality and potentially hindering the visibility of anatomical structures (e.g. valves, small-sized vessels, aortic dilatation)4,5. Previous work demonstrated that ultra-short TE (UTE) sequences are effective in minimizing flow-related dephasing5,6. However, in absence of a contrast agent, TE shortening comes at the expense of blood-to-myocardium contrast, negatively affecting anatomical visualization and reliable SG signals extraction (previously addressed by acquiring a second echo at longer TE for self-navigation, at the cost of scan time)7.We tested the hypothesis that, by integrating a 3D radial UTE acquisition7 within the free-running framework1 in ferumoxytol-enhanced MRI of congenital heart disease (CHD), the inherent advantages (absence of flow artifacts and excellent contrast) can mutually compensate the inherent disadvantages (low contrast and presence of flow artifacts) of the two components. This simultaneously allows for self-navigation within a single-echo UTE acquisition and yields flow artifact-free 5D images with improved visibility of anatomical structures.
Methods
Acquisitions. Seven CHD patients (age: 3-38 y; 5 male) underwent imaging on a 1.5T MAGNETOM Sola (Siemens Healthcare, Erlangen, Germany), after providing IRB-approved written informed consent. For each patient, ferumoxytol-enhanced whole-heart data were acquired with a prototype free-running UTE sequence7 with an option for multi-echo readouts (TE=0.08ms, n=4 with dual- and n=3 with single-echo UTE, see Fig.1) as well as with a previously reported version for free-running GRE2. With equal acquisition time TAUTE=5:20min, single-echo UTE had a 54% increase in scanning efficiency as compared to dual-echo UTE. For each dataset and echo, SG signals were extracted and used for data sorting into motion-resolved bins (4 respiratory, 50ms cardiac)1.UTE self-gating. To test the hypothesis that the increase in contrast with ferumoxytol allows for reliable SG signals extraction from single-echo UTE acquisitions, SG signals obtained from the two echoes of the dual-echo UTE acquisitions (n=4) were compared8. For cardiac SG, the time between consecutively detected triggers (RR intervals) was compared by means of a Bland-Altman analysis and paired-sample t-tests. The similarity between SG respiratory signals was evaluated via the Pearson correlation coefficient, while their concordance in terms of assignments of readouts to respiratory bins was quantified by measuring the percentage of coinciding assignments.
Reconstructions. 5D UTE and 5D GRE images were reconstructed1. End-expiratory lung-liver sharpness9 was compared (paired-sample Wilcoxon signed rank test), and the quality of motion-resolution was qualitatively assessed by means of M-mode images.
Flow artifacts in UTE and GRE reconstructions. To test the hypothesis that UTE helps minimizing flow-related dephasing, the severity of flow artifacts in 5D UTE and 5D GRE images was evaluated by computing the standard deviation of the mean signal intensity across cardiac phases in a ROI covering a cross-section of the ascending aorta.
Results
UTE self-gating. Good agreement was found between SG signals obtained from the two echoes of dual-echo UTE acquisitions. For cardiac SG, Bland-Altman analysis of RR intervals revealed low bias and good limits of agreement (0.08±32.65 ms) and t-tests did not reveal statistically significant differences (pttest>0.05) albeit in a small cohort (Fig.2A-B). Respiratory SG signals were also comparable, both in terms of signal correlation (rPearson=0.94±0.05) and binning (coincidence of 82.6%, 70.1%, 75.4% and 90.1% from end-expiration to end-inspiration), with mismatches mostly due to assignments to neighboring bins (Fig.2C-D).Reconstructions. Overall, 5D UTE and 5D GRE reconstructions displayed good delineation of cardiac anatomy, albeit with more blur (sUTE=2.10±0.21, sGRE=2.55±0.36, pWilcoxon<0.05, n=7) in 5D UTE (Fig.3A and Fig.5). M-mode images suggested a comparable level of motion-resolution (Fig. 3B).
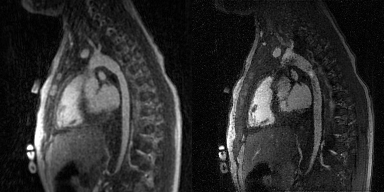
Flow artifacts in UTE and GRE reconstructions. Visual inspection of 5D images revealed no flow artifacts in 5D UTE images, while they appeared prominent on 5D GRE, affecting the visibility of anatomical structures (Fig.4A and Fig.5). This was corroborated by quantitative measures (Fig.4B).
Discussion and conclusion
In this work, we showed the benefits of integrating a 3D radial UTE acquisition within the free-running framework in ferumoxytol-enhanced MRI. On one hand, we confirmed our first hypothesis that the increased blood-to-myocardium contrast offered by ferumoxytol allows for robust self-navigation based solely on the UTE echo, resulting in time-efficient acquisitions offering potential for increased sampling or reduced acquisition time. On the other hand, we confirmed our second hypothesis that the TE shortening enabled by UTE acquisitions allows for a significant reduction of signal dephasing, yielding flow artifact-free 5D images with improved visibility of dynamic anatomical structures. Additional work on the acquisition (protocol, trajectory optimization) and on the reconstruction side (reconstruction parameters, gradient imperfections correction) is still needed to bring the framework to realize its full potential in the context of ferumoxytol-enhanced imaging of CHD patients.Acknowledgements
The work was funded by the Swiss National Science Foundation (SNSF 320030B_201292)References
[1] Di Sopra, L., Piccini, D., Coppo, S., Stuber, M., & Yerly, J. (2019). An automated approach to fully self‐gated free‐running cardiac and respiratory motion‐resolved 5D whole‐heart MRI. Magnetic resonance in medicine, 82(6), 2118-2132.
[2] Di Sopra, L., Roy, C. W., Whitehead K. K., Fogel M. A., Piccini, D., & Stuber, M. Study on the potential acceleration of ferumoxytol-enhanced free-running 5D whole-heart MRI in pediatric patients. Proc. Intl. Soc. Mag. Reson. Med. , 749550 (2020).
[3] Heerfordt, J., Whitehead, K. K., Bastiaansen, J. A., Di Sopra, L., Roy, C. W., Yerly, J., ... & Piccini, D. (2021). Similarity‐driven multi‐dimensional binning algorithm (SIMBA) for free‐running motion‐suppressed whole‐heart MRA. Magnetic Resonance in Medicine, 86(1), 213-229.
[4] Monney, P., Piccini, D., Rutz, T., Vincenti, G., Coppo, S., Koestner, S. C., ... & Schwitter, J. (2015). Single centre experience of the application of self-navigated 3D whole heart cardiovascular magnetic resonance for the assessment of cardiac anatomy in congenital heart disease. Journal of Cardiovascular Magnetic Resonance, 17(1), 1-12.
[5] Robson, M. D., Gatehouse, P. D., Bydder, M., & Bydder, G. M. (2003). Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. Journal of computer assisted tomography, 27(6), 825-846.
[6] Herrmann, K. H., Krämer, M., & Reichenbach, J. R. (2016). Time efficient 3D radial UTE sampling with fully automatic delay compensation on a clinical 3T MR scanner. PLoS One, 11(3), e0150371.
[7] Delacoste, J., Chaptinel, J., Beigelman‐Aubry, C., Piccini, D., Sauty, A., & Stuber, M. (2018). A double echo ultra-short echo time (UTE) acquisition for respiratory motion‐suppressed high-resolution imaging of the lung. Magnetic resonance in medicine, 79(4), 2297-2305.
[8] Falcão, M. B., Di Sopra, L., Ma, L., Bacher, M., Yerly, J., Speier, P., ... & Roy, C. W. (2021). Pilot tone navigation for respiratory and cardiac motion‐resolved free‐running 5D flow MRI. Magnetic resonance in medicine, 00:1-15.
[9] Ahmad, R., Ding, Y., & Simonetti, O. P. (2015). Edge sharpness assessment by parametric modeling: application to magnetic resonance imaging. Concepts in Magnetic Resonance Part A, 44(3), 138-149.
[10] Piccini, D., Littmann, A., Nielles‐Vallespin, S., & Zenge, M. O. (2011). Spiral phyllotaxis: the natural way to construct a 3D radial trajectory in MRI. Magnetic resonance in medicine, 66(4), 1049-1056.
Figures
Figure 1. UTE sequence. A: RF pulses are followed by center-out UTE readouts using ramp-sampling. A second full-echo can be acquired for SG. B-C: The spiral phyllotaxis trajectory10 was adapted to ensure full k-space coverage. In our work, sequence parameters were: RF excitation angle=15°, base resolution=192, spatial resolution=(1.14mm)3, TE1/TE2=0.08/2.17ms, half readouts=81906 for dual- and 126478 for single-echo UTE, resulting in constant acquisition time TAUTE=5:20min with 54% more sampling for single-echo UTE. For reference TAGRE=5:59 min (full readouts=126478).
Figure 2. Comparison of SG signals obtained from the first (E1) and second (E2) echoes of dual-echo UTE acquisitions. A: Bland-Altman analysis of RR intervals from E1 and E2. B: RR intervals (mean±std) from E1 and E2 are reported with the results of paired-sample t-tests. For reference, RR intervals from GRE are reported. C: Examples of SG respiratory signals from E1 and E2. D: Assignments of readouts to respiratory bins (EE: end-expiration, ME: mid-expiration, MI: mid-inspiration, EI: end-inspiration) based on SG respiratory signals from E1 and E2 (green: coinciding assignments).
Figure 4. Flow artifacts in 5D UTE and 5D GRE images. A: Comparison of flow artifacts (dashed line: aortic valve; circle: aortic cross section; see Fig.3A) for different cardiac phases (CP) for a 5D UTE (top, green) and the corresponding 5D GRE (bottom, pink) reconstructions. B: Evolution of mean signal intensity for a ROI on the aorta for a 5D UTE (top: dual-echo, dataset shown in A; bottom: single-echo) and the corresponding 5D GRE (pink) reconstructions. C: Standard deviation of mean signal intensity over CP across all patients (from left to right: dual-echo UTE, single-echo UTE, GRE).