0328
Gadolinium-free Bright and Black-Blood 3D whole-heart MRI for efficient anatomical assessment in patients with Congenital Heart Disease1Biomedical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, London, United Kingdom, 4Guy’s and St Thomas’s Hospital, London, United Kingdom, 5Escuela de Ingeniería, Pontificia Universidad Católica de Chile, Santiago, Chile
Synopsis
A recently developed sequence, the Magnetisation Transfer Contrast Bright Blood phase SensiTive (MTC-BOOST), enables free-breathing simultaneous acquisition of a bright-blood and black-blood 3D whole-heart dataset. We sought to compare the acquisition time, image quality and diagnostic performance of the proposed MTC-BOOST against the native clinical T2-prep bSSFP and the contrast-enhanced (CE) T2-prep bSSFP sequence in a cohort of patients with Congenital Heart Disease. Our study showed that MTC-BOOST offers good delineation of the intracardiac and vascular anatomy that is comparable to the clinical CE T2prep bSSFP and superior to the non-CE T2-prep bSSFP while offering a shorter acquisition time.
Background
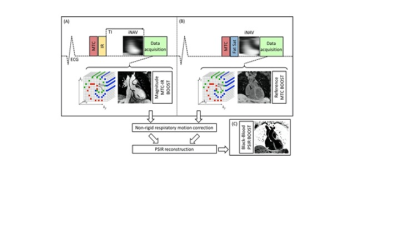
Bright- and black-blood whole heart (WH) imaging sequences are utilised for the morphological evaluation of patients with Congenital Heart Disease (CHD). Current clinical approaches commonly include a 2D breath-hold Half-Fourier Acquisition Single-shot Turbo spin Echo (HASTE)1 for black-blood visualisation along with a 3D T2-prepared balanced Steady State Free Precession (T2-prep bSSFP)1 bright-blood sequence. Limitations include patient discomfort due to multiple breath-holds and 2D anatomic visualisation for the HASTE sequence, and long scan times due to diaphragmatic navigators as well as flow and off-resonance related image artefacts2 for the T2prep-bSSFP sequence. Gadolinium based contrast agents are often employed to enhance the image quality of T2prep-bSSFP, albeit with associated side effects. We have recently proposed a sequence that enables simultaneous acquisition of 3D bright- and black-blood motion compensated images under free-breathing, by exploiting Magnetisation Transfer Contrast (MTC-BOOST)3 for high-quality depiction of both the arterial and the venous systems without the need of contrast injection. It utilises MTC-IR preparation pulse in odd heartbeats to achieve sufficient contrast of the blood pool and the myocardium. A MTC solely acquisition in even heartbeats is employed to allow sufficient time for magnetisation recovery; and through Phase Sensitive Inversion Recovery Reconstruction of the two bright-blood datasets, a complementary black-blood dataset is generated (Fig. 1). The aim of this study is to validate the MTC-BOOST against the native clinical T2-prep bSSFP and contrast-enhanced (CE) T2-prep bSSFP sequences with respect to the visualisation of intracardiac and vascular structures in patients with CHD and to investigate potential merits of the complementary black-blood dataset.Methods: Data acquisition
An MTC-BOOST prototype sequence was evaluated in 40 patients (32 ±11 years old, 22 Female) with CHD on a 1.5T system (MAGNETOM Aera, Siemens Healthcare). Acquisition was performed with 3-fold undersampling with a Cartesian trajectory with spiral profile order4 and 2D image-based navigation5 was utilised to enable beat-to-beat translational and bin-to-bin non-rigid respiratory motion and 100% respiratory scan efficiency. The undersampled non-rigid motion-corrected reconstruction framework was implemented in-line in the scanner. Imaging parameters included: free-breathing with no respiratory gating, subject-specific mid-diastolic ECG-triggering, coronal orientation, resolution 1.5mm3 isotropic, FOV 320x320x156-177mm3,flip angle 90°, TE/TR 1.35/3.2ms, 3-fold undersampling, MT preparation:15 Gaussian pulses, flip angle 800, frequency offset 3000Hz, duration 20.5ms. As part of the clinical protocol 30 patients underwent native 3D T2-prep bSSFP and 10 patients underwent CE T2-prep bSSFP (FOV 400x300x155-165mm3, spatial resolution 1.5mm3, T2-prep duration = 40 ms, GRAPPA parallel imaging 2x undersampled, flip angle = 90˚, sagittal orientation).Methods: Data analysis
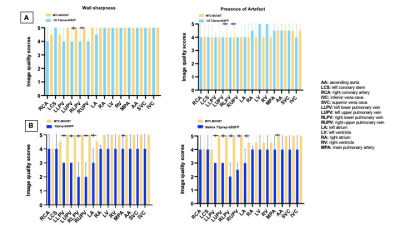
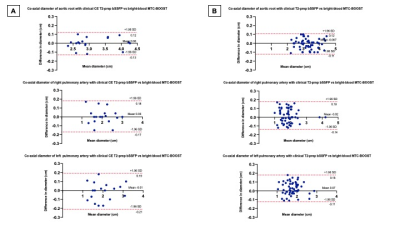
Acquisition time for the clinical and the MTC-BOOST dataset was recorded. Two experts blinded to the technique used, recorded their diagnostic confidence (≥ 3: full diagnostic confidence). One expert blinded to the acquisition method assessed image quality scores for 14 structures using 5-point Likert scale (3≥ diagnostic). Co-axial vascular dimensions at the level of the aortic root, right and left pulmonary arteries were obtained with both methods and compared by the same blinded reader using Bland-Altman analysis.Results
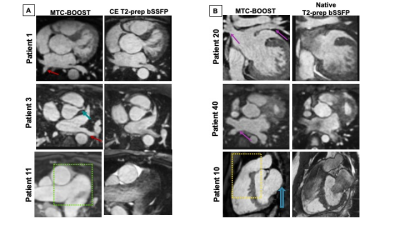
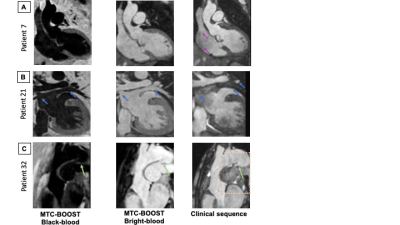
Good quality depiction of all intrapericardiac structures (Fig. 2 and 3) was achieved with MTC-BOOST in short scan time [Clinical sequence: 14 ±7min vs 10 ±4 min, p<0.0001]. Diagnostic confidence was higher for the MTC-BOOST bright-blood than for the clinical T2-prep bSSFP [4(4,4) vs 4(3,4), p=0.0019 respectively] and similar to CE clinical sequence [4(4,4) vs 4(4,4) p>0.99 ]. Image quality scores for the assessed vascular structures were consistently high in the MTC-BOOST bright-blood dataset with marked superiority of the MTC-BOOST sequence in the delineation of pulmonary veins. The great arteries and systemic veins are also better visualized with the MTC-BOOST when compared with the native T2-prep bSSFP (Fig. 4). MTC-BOOST resulted in improved luminal signal and was robust towards rapid, turbulent flow and off-resonance effects (Fig. 2 & 3). The complementary 3D black-blood volume of the MTC-BOOST did not result overall in significant improvement in the diagnostic confidence compared to solely utilising the bright-blood MTC-BOOST dataset [4(4,4) vs 4(4,4), p=>0.99] (Fig. 3). Bland-Altman analysis demonstrated very good agreement in the co-axial aortic measurements of the aortic root, RPA and LPA between the MTC-BOOST and the corresponding clinical T2-prep bSSFP or CE T2-prep bSSFP dataset, with a mean difference of <0.07 cm across these landmarks (Fig. 5).Discussion
The proposed MTC-BOOST sequence offers good depiction of the intracardiac and vascular anatomy that is comparable to the clinical CE T2prep bSSFP and superior to the non-CE T2-prep bSSFP sequence in a shorter scan time than the clinical standard. Flow and off-resonance artefacts are attenuated with the proposed approach. The proposed method may obviate the need for gadolinium-based contrast agents in this patient group and promises to be beneficial in clinical practice. An additional clinical value of the black-blood complementary dataset has only been demonstrated in a single case in this patient group. It is hypothesised though that it can potentially enable visualisation of thrombus (Fontan circulation, venous baffles).Conclusion
The MTC-BOOST sequence offers superior quality 3D-WH imaging to the conventional native T2-prep bSSFP sequence and is equivalent to the diagnostic performance of the CE T2-prep bSSFP in shorter acquisition time. It has potential to enhance the time- and cost-effectiveness of bright-blood 3D-WH imaging in patients with CHD.Acknowledgements
No acknowledgement found.References
1.Fratz S, Chung T, Greil GF, et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. Journal of cardiovascular magnetic resonance. 2013;15(1):51.
2.Hu P, Stoeck CT, Smink J, et al. Non-contrast SSFP pulmonary vein MRA: Impact of off-resonance and flow. Journal of magnetic resonance imaging. 2010;32(5):1255-1261.
3.Ginami, G., Lòpez, K., Mukherjee, R., Neji, R., Munoz, C., Roujol, S., Mountney, P., Razavi, R., Botnar, R. and Prieto, C., 2018. Non‐contrast enhanced simultaneous 3D whole‐heart bright‐blood pulmonary veins visualization and black‐blood quantification of atrial wall thickness. Magnetic Resonance in Medicine, 81(2), pp.1066-1079.
4.Prieto, C., Doneva, M., Usman, M., Henningsson, M., Greil, G., Schaeffter, T. and Botnar, R., 2014. Highly efficient respiratory motion compensated free-breathing coronary mra using golden-step Cartesian acquisition. Journal of Magnetic Resonance Imaging, 41(3), pp.738-746.
5.Henningsson M, Koken P, Stehning C, Razavi R, Prieto C, Botnar RM. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn Reson Med. 2012;67(2):437-445. doi:10.1002/mrm.23027
Figures